Spatial smelling
The term spatial smelling , also known as stereoscopic , is understood as the direction of a fragrance source being recognized by an organism . In principle, the localization of an odor source is possible in two different ways: in a clinotactic way, i.e. by comparing temporally offset information from a receptor / sensory organ, or tropotactically , which corresponds to a symmetrical orientation towards the stimulus, for which two spatially separate sensory organs are necessary. In the latter case, one speaks of spatial smelling . Although, for example, all insects and almost all mammals , including humans , meet the physiological requirements of two spatially separated olfactory organs, this ability has so far only been clearly demonstrated experimentally in a very small number of animal species . For physical reasons, single-cell organisms are not able to “smell” spatially.
meaning

All animals , from unicellular prokaryotes to humans, orient themselves using chemical stimuli from their environment. The neural comparison of signals received bilaterally , i.e. bilaterally , is the basis for the ability of visual space perception , spatial vision and the localization of noises in space, spatial hearing in organisms. This is made possible by two eyes or two ears. This constellation is also given for smelling in most vertebrates (Vertebrata) and very many invertebrates (Invertebrata), since almost all of these organisms also have two "sensors", for example nostrils (nares) or antennae , which serve the sense of smell . All sub-trunks of the arthropods - with the exception of the jaw-claw bearers (Chelicerata) - have the biological prerequisites for spatial smelling; all trachea (Tracheata), also called Antennata (antenna carrier), and crustaceans (Crustacea). With the exception of the toothed whale , all mammals have two nostrils. In the course of evolution, toothed whales have formed one 'nostril', the blowhole , from two nostrils , while all baleen whales have two 'nostrils'.
While the contribution and function of the eyes and ears in spatial perception are clear, this is still completely unclear for many animal species in the case of two odor sensors or two separate olfactory channels, which in principle enable spatial smelling. The ability of spatial smelling has only been clearly demonstrated in very few species, such as the desert ant Cataglyphis fortis , the East American mole ( Scalopus aquaticus ) and in pet rats ( Rattus norvegicus forma domestica).
Spatial Smelling Principles
The information 'smell' or 'something smells there' is almost worthless for the smelling organism in the struggle for existence . Without localization of odor source can, for example, his food or his sexual partner can not find or its predator ( predator ) not escape unless other sense organs fulfill this task adequately.
The localization of an odor source by an organism is basically possible in two ways. By serial or parallel detection of the fragrance. In serial sampling , the olfactory organ, for example the nose, is moved to different places and the difference in smell between the two places is compared. The position at which the stronger odor impression was obtained is obviously closer to the odor source. The axis, which results geometrically from the two sniffing points in the room, points at least roughly in the direction of the odor source. By further serial smelling in the direction of this axis, the odor source can be localized more closely and, if necessary , clearly localized by other senses (sight, touch ). Serial smelling is a form of clinotaxis, that is, orientation by comparing information from a receptor / sensory organ that has been staggered in time. In the case of parallel detection ( bilateral nasal cues = 'bilateral nasal stimuli'), the localization of the fragrance takes place simultaneously via two spatially separate olfactory channels or sensors. In almost all mammals, these are the two nostrils. The difference in intensity, which can be determined directly via the separate channels, corresponds to an odor gradient, which in turn can be used to locate the direction. When hearing, in the case of one-sided deafness, serial smelling corresponds to turning the head to locate the sound source . The parallel sniffing then corresponds to spatial hearing with two ears, in which the difference in transit time between the two ears has a significant share in the directional localization of the sound source. The parallel smell is in turn a form of tropotaxis, the symmetrical alignment to the stimulus, for which two spatially separated sensory organs are necessary.
The parallel detection has several advantages over the serial one. The direct comparison is much faster in terms of time, in the case of mammals it can be done in one breath, as it were. In addition, it is - similar to a differential amplifier in electronics - significantly more sensitive. With the serial process there is also the risk that parts of the receptors are still blocked by the first smell during the second smell.
The tracking search (Engl. Scent-tracking ), for example by a Fährten- or bloodhound , can in principle klinotaktisch, and carried tropotaktisch. It could be shown experimentally that the tropotactic compared to the clinotactic tracking search brings with it considerable time advantages.
Protozoa and sperm
Chemotaxis is the influencing of the direction of movement of organisms by a substance concentration gradient. In unicellular organisms, chemotaxis is the simplest form of smell or taste detection and one of the most fundamental physiological cell reactions. It is of great importance for the survival of protozoa and for a large number of physiological processes. It is used, for example, to locate beneficial, but also harmful substances. For example, nutrients are beneficial, while toxins are harmful substances. Human sperm, for example, follow the attractant progesterone that the egg cell releases. In addition , sperm express over 30 different olfactory receptor genes . One of them is the olfactory gene OR1D2 , which is also expressed in the olfactory mucosa . Mutations in OR1D2 may affect not only the ability to perceive the smell of the fragrance bourgeonal , but also the fertility of the affected man. Bourgeonal has a lily of the valley- like odor. The odor threshold is significantly lower in men than in women. It is the only fragrance known to date with a gender-specific difference in the odor threshold. The function of the olfactory receptors of the sperm cells in the fertilization process is controversial. Regardless of what the chemotaxis causes and how the attractant or deterrent is perceived, at the level of a single cell, localization is always carried out through a serial process. In the immediate vicinity of a cell, due to its small size (typically in the range from 1 to 10 µm) and the Brownian movement , there is no concentration gradient ; the concentration is isotropic . It is therefore not possible for a stationary single-cell organism to use receptors to recognize the direction of an attractant or deterrent at two distant points on its surface. Free-swimming, flagellated bacteria such as Salmonella Typhimurium solve this problem by first swimming a distance in a randomly determined direction. If the concentration of the attractant detected by the receptors increases, they continue to swim in this direction. If it decreases, the direction is changed. In the case of terrible substances, they behave the other way around.
insects

In insects, the antennae (antennae) form the olfactory organs. Examples are bees and moths . With these paired antennas, a simultaneous odor perception is possible with some insect species via the olfactory senses on them. The odor location can be localized via the odor and time gradient. For example, the location of a flower or that of a potential sexual partner who releases pheromones can be determined. If an antenna is lost, these insects move in a circle, always in the direction of the remaining antenna. In 1910 the Swiss Auguste Forel (1848–1931) was the first to postulate a spatial smell in ants. In desert ants of the species Cataglyphis fortis , this ability could be demonstrated for the first time exactly 100 years later. These ants spatially smell their surroundings, for which they need both antennas. In addition, they use the distribution of different scents in the nest environment, similar to a map, for navigation. Evidence of these abilities was demonstrated in Cataglyphis fortis with two experiments . The nest entrance was marked with the four fragrances salicylic acid methyl ester , decanal , nonanal and indole in a specific pattern and the ants trained on it. If this odor pattern was shifted locally, the animals followed it, assuming that their nest was there. If the scent pattern was changed, the ants lost their orientation. The thesis derived from these results that two separate organs of perception - here antennas - are necessary for this ability, similar to vision, was confirmed in the second experiment. Ants with only one antenna could no longer orientate themselves.
Crustaceans
The Caribbean lobster ( Panulirus Argus ) has the second and third Tagma their head - like all crustaceans - one pair of antennas. The smaller antennae on the second tagma are called antennae ('first antennae'). The aesthetic tubes (olfactory tubes, cuticular sensillae ) sit in a row on these antennas . They contain the olfactory sensory cells that serve to perceive water-soluble fragrances. As an inhabitant of the sea floor , the Caribbean lobster, like all decapods , is dependent on the detection and localization of scents in their habitat. These chemical signals control a multitude of behaviors, for example interactions with conspecifics, the escape of predators, the choice of hiding place, the cleaning behavior and the perception and location of food. In the American rust crayfish ( Orconectes rusticus ) it could be shown that it needs both antennas for orientation. It makes no difference whether only one or both antennae have been removed - the orientation is equally bad in both cases.
In the Caribbean lobster, each aesthetic mask is innervated by the dendrites of around 300 olfactory receptor neurons , via whose axons the signals are sent to the olfactory glomeruli of the olfactory lobes. For a long time it was assumed that the aesthetic masks were the most important sensors for recognizing, differentiating and locating fragrances. A number of studies also show that removing the antennas has an influence on the fragrance-related behavior of the Caribbean lobster. The lobster uses both tropotactic and clinotactic comparisons of odor intensities to localize scents. Recent studies on the Caribbean spiny lobster have shown that the aesthetasques are not the only sensors on the two antennas that these animals use to locate their food.
Mammals
Brown rats
The distance between the nostrils in brown rats is about 3 mm. In an analysis of the flow conditions in the nose carried out in 1999, it was found that, despite this comparatively small distance, rats inhale the air to the side of each nostril and thus separately. The overlap of the air flows when breathing in and out is very slight. Together with the presence of spatial receptive fields that are specifically arranged to the side, these findings were used to hypothesize that rats are in principle able to carry out independent bilateral odor comparisons via both nostrils. The separate air currents, together with the separate axonal projections from the olfactory mucosa into the olfactory bulb, are the basic requirements for this ability. On the basis of these findings, an Indian working group investigated the abilities of odor detection in Wistar rats in 2006 . They determined the sniffing frequency of the test animals at 7 to 8 Hz , which means that a rat 'smells' seven to eight times per second. In further experiments, the experimenters used 2-phenylethanol as a fragrance that does not stimulate the trigeminal nerve . Within about 0.125 seconds, i.e. with a single sniff, the rats could see the direction of the 2-phenylethanol. With a comparative, serial sniff, the rats would have needed at least twice the time for this. The authors of the study conclude that with each sniff, rats receive a complete olfactory snapshot of their environment, which includes both the identity and the location of the odor.
East American mole
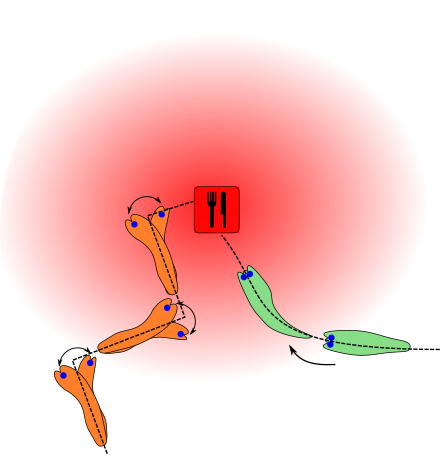
The East American mole ( Scalopus aquaticus ) has degenerated mechanoreceptive organs compared to other moles . The very small eyes are covered by skin and fur and the ears, which are also very small, are tuned to low frequencies. These comparatively underdeveloped sensory organs are hardly considered for the localization of prey. Nevertheless, the East American mole can move quickly and directly towards its prey, seemingly sniffing serially as it moves. To check whether the odor detection actually takes place serially or at least in parallel, a test chamber with several defined feeding places was built in a much-noticed study in 2012 (see sketch). With a special pressure sensor and a high-speed camera , East American moles were observed foraging for food. For this purpose, nasal adapters in the form of short polyethylene tubes were attached to them. With this adapter the air flow could be changed. If the tubes were designed in a straight line so that the right end of the tube led to the right nostril and, accordingly, the left to the left nostril, the test animals were able to find their food quickly and without change. If, on the other hand, the two tubes were crossed so that the right end of the tube supplied the left nostril with air and the left the right nostril, they could no longer find their food, but usually moved in exactly the opposite direction of the food source. If one nostril was blocked in the moles, they were able to locate their food, but this required significantly more time than with two free nostrils. The author of the study concludes from his results that parallel smelling is of great importance, especially at close range. In the vicinity of the fragrance source, the odor gradient is significantly greater than in the distance, which provides faster and better information about the location of the source when comparing the intensity between the two nostrils. From this he derives a hypothetical search strategy that consists of two components. In the distance, with low odor gradients, large movements are made with serial smelling in different places to localize the direction. In the vicinity of the fragrance source, i.e. with a larger odor gradient, the movements are shorter and the bilateral comparison between the two nostrils is in the foreground. The significantly poorer odor detection when closing a nostril could, however, also be due to another problem. The occlusion may lead to a constant pull ( bias ) of the animal in the direction of the open nostril.
human
Whether a person is able to smell spatially - possibly only through intensive training - is controversial. Basically, it used to be assumed that the distance between the two nostrils in almost all mammals, and especially in humans, was not sufficient to obtain spatially separated information that is necessary for spatial smelling. The sense of smell also plays only a subordinate role in humans, for example in comparison to a rat.
In humans, as in most other mammals, the sense of smell is conveyed via two nerves: via the olfactory nerve ( Nervus olfactorius ) and via the Nervus trigeminus . The olfactory nerve has the largest share in the olfactory sensation . It guides the olfactory impression of a large number of fragrances, for example that of vanillin or rotten eggs ( hydrogen sulfide ), from the olfactory mucous membrane ( Regio olfactoria ) to the primary olfactory cortex of the cerebrum ( Telencephalon ). The substances mentioned in the examples are pure fragrances in low concentrations that only stimulate the olfactory nerve. In fact, almost all fragrances also stimulate the trigeminal nerve , which therefore plays a major role in the perception of smells. On the other hand, there are substances that are only able to irritate the trigeminal nerve . An example of this is carbon dioxide , an odorless gas which, in higher concentrations , is perceived by the trigeminal nerve as "sour", "tingly". The trigeminal nerve has a protective or defense function against a large number of irritating or toxic substances. The trigeminal stimulation leads to sensations such as “burning”, “cooling” and “tingling”, even in the absence of olfactory perception.
Georg von Békésy , the Hungarian-American Nobel Prize laureate for Physiology or Medicine , recognized some analogies between hearing and smelling in humans. In 1964 he published the results of his own studies. Among other things, he found that a difference in transit time in the order of 0.1 milliseconds between the two nostrils is registered and can be used to determine the direction. This is a time value similar to that of listening. From this he calculated an angular range of 7 to 10 °, with which the odor source can in principle be located. He used benzene , cloves , lavender oil and eucalyptus oil as fragrances . In doing so, he found no differences in these fragrances with regard to the odor localization ability.
In January 2007, a study was published in which the 32 study participants had to follow a 10 m long scent trail made of chocolate oil under various test conditions outdoors. In all experiments, the other sensory organs were largely switched off by glasses, hearing protection and gloves. Two thirds of the subjects were able to follow the scent trail. Four of the capable participants were trained to search for tracks for several days. The speed of the track search could be more than doubled. The speed in turn correlated directly with the sniffing frequency. When analyzing the air currents in the area of the nose during sniffing, or nasal breathing, the authors made a surprising discovery: Each nostril draws air from different, non-overlapping areas in the room. The spatial resolution of both nostrils calculated from the flow tests is about 35 mm. Assuming that the border area of a scent cloud is approximately 10 mm wide, one nostril can be inside and one outside of the scent cloud when following a scent trail. From a purely physical point of view, it should be possible for humans to smell in space. To check this, one of the subjects' nostrils was closed in a further series of experiments. As a result, the search for tracks was significantly less accurate and significantly slower than with both nostrils. If the test subjects were fitted with a nasal adapter in which the air for both nostrils was sucked in separately, but mixed in front of the nose and then divided again so that the spatial resolution is lost, the results of the tracking search were as bad as with one nostril. With a nasal adapter in which the two air currents were not mixed in front of the nostrils, the test subjects were 24% faster. From these results, the authors concluded that
- People are in principle able to follow a scent trail,
- this ability can be significantly improved through training,
- the spatial resolution of the human nose is in the range of 35 mm and
- the search for tracks is supported by an internasal odor comparison (spatial smell).
Other studies seem to contradict these conclusions. They explain that trigeminal stimulation is necessary to localize an odorous substance . In 1989 tests with pure fragrances such as hydrogen sulfide or vanillin, it was found that with this purely olfactory stimulation the test persons were not able to smell spatially. The results of the experiment turned out to be completely different when fragrances were to be located that also cause trigeminal stimulation, that is, that can also be “tasted”. These include, for example, carbon dioxide or menthol . This applies to both children and adults. Humans are able to localize trigeminal and olfactory-trigeminal stimuli, while this is obviously not or hardly possible with fragrances that trigger purely olfactory stimuli.
further reading
- Thomas Hummel, Antje Welge-Lüssen (eds.): Taste and Smell. Volume 63 of Advances in oto-rhino-laryngology. Karger Medical and Scientific Publishers, 2006, ISBN 3-8055-8123-8 , limited preview in Google book search.
- Donald A. Wilson, Richard J. Stevenson: Learning to Smell: Olfactory Perception from Neurobiology to Behavior. JHU Press, 2006, ISBN 0-8018-8368-7 , limited preview in Google Book Search.
- S. Kikuta, K. Sato, et al. a .: From the Cover: Neurons in the anterior olfactory nucleus pars externa detect right or left localization of odor sources. In: Proceedings of the National Academy of Sciences of the United States of America . Volume 107, number 27, July 2010, ISSN 1091-6490 , pp. 12363-12368, doi: 10.1073 / pnas.1003999107 , PMID 20616091 , PMC 2901466 (free full text).
Web links
- Video about the effects of one-sided nostril blockage in the East American mole (top: left nostril blocked, middle: both nostrils open, bottom: right nostril blocked; material for publication below)
- Video about the effects of a nostril adapter on the East American mole first with a "normal" adapter (right nostril is moved to the right, left nostril to the left), then with a "crossed" adapter (right nostril is moved to the left, left to the right; material for publication below )
- Veronika Szentpétery: Rats smell in stereo. At: Telepolis on February 3, 2006
Individual evidence
- ^ A b Peter M. Kappeler: Behavioral Biology. Springer, 2006, ISBN 3-540-24056-X , pp. 113-114.
- ↑ a b c M. Louis, T. Huber u. a .: Bilateral olfactory sensory input enhances chemotaxis behavior. In: Nature Neuroscience . Volume 11, Number 2, February 2008, ISSN 1097-6256 , pp. 187-199, doi: 10.1038 / nn2031 , PMID 18157126 .
- ↑ a b c d e f g h K. C. Catania: Stereo and serial sniffing guide navigation to an odor source in a mammal. In: Nature Communications . Volume 4, 2013, ISSN 2041-1723 , p. 1441, doi: 10.1038 / ncomms2444 , PMID 23385586 . ( Open Access , CC BY-NC-SA 3.0)
- ^ A b Friedrich Wilhelm Merkel , M. Walter Schäfer : Orientation in the animal kingdom. Fischer, 1980, ISBN 3-437-20221-9 , p. 9.
- ↑ a b c J. Porter, B. Craven et al. a .: Mechanisms of scent-tracking in humans. ( Memento from April 25, 2013 in the Internet Archive ) In: Nature Neuroscience . Volume 10, Number 1, January 2007, ISSN 1097-6256 , pp. 27-29, doi: 10.1038 / nn1819 , PMID 17173046 .
- ↑ C. Brenker, N. Goodwin et al. a .: The CatSper channel: a polymodal chemosensor in human sperm. In: The EMBO Journal . Volume 31, number 7, April 2012, ISSN 1460-2075 , pp. 1654-1665, doi: 10.1038 / emboj.2012.30 , PMID 22354039 , PMC 3321208 (free full text).
- ↑ Hans Hatt : Taste and smell. In: Robert F. Schmidt , Florian Lang (Hrsg.): Physiologie des Menschen. 30th edition. Springer, 2007, ISBN 978-3-540-32908-4 , pp. 421-436.
- ↑ G. Ottaviano, D. Zuccarello et al. a .: Human olfactory sensitivity for bourgeonal and male infertility: a preliminary investigation. In: European archives of oto-rhino-laryngology. Volume 270, Number 12, November 2013, ISSN 1434-4726 , pp. 3079-3086, doi: 10.1007 / s00405-013-2441-0 , PMID 23525651 .
- ↑ P. Olsson, M. Laska: Human male superiority in olfactory sensitivity to the sperm attractant odorant bourgeonal. In: Chemical senses. Volume 35, Number 5, June 2010, ISSN 1464-3553 , pp. 427-432, doi: 10.1093 / chemse / bjq030 , PMID 20378596 .
- ↑ T. Strünker: The end of the "lily of the valley phenomenon" in sperm research? Max Planck Society, dated February 24, 2012
- ^ RM Macnab, DE Koshland: The gradient-sensing mechanism in bacterial chemotaxis. In: PNAS . Volume 69, Number 9, September 1972, ISSN 0027-8424 , pp. 2509-2512, PMID 4560688 , PMC 426976 (free full text).
- ^ J. Adler, WW Tso: "Decision" -making in bacteria: chemotactic response of Escherichia coli to conflicting stimuli. In: Science . Volume 184, Number 4143, June 1974, ISSN 0036-8075 , pp. 1292-1294, PMID 4598187 .
- ^ Gilbert Waldbauer: What Good Are Bugs? Insects in the Web of Life. Harvard University Press, 2009, ISBN 978-0-674-04474-6 , p. 19 limited preview in Google book search
- ↑ A. Forel: The sensory life of insects: a collection of experimental and critical studies on insect psychology. E. Reinhardt, Munich, 1910.
- ↑ Karl Gößwald : The wood ant: Biological foundations, ecology and behavior. Aula, 1989, ISBN 3-89104-475-5 , p. 404.
- ↑ a b Kathrin Steck: Smells like home: Olfactory landmarks in desert ant orientation. Dissertation, Friedrich Schiller University Jena, 2010.
- ↑ K. Steck, M. Knaden, BS Hansson: Do desert ants smell the scenery in stereo? In: Animal Behavior. Number 4, Volume 79, 2010, pp. 939-945, doi: 10.1016 / j.anbehav.2010.01.011
- ^ Bill S. Hansson, K. Steck, M. Knaden: Scent Landscape in Stereo. Max Planck Society, March 9, 2010
- ↑ Aesthetic Masks. In: Lexicon of Neuroscience. Spectrum Academic Publishing House, 2000
- ↑ J. Atema: Chemical signals in the marine environment: dispersal, detection, and temporal signal analysis. In: Proceedings of the National Academy of Sciences of the United States of America . Volume 92, Number 1, January 1995, ISSN 0027-8424 , pp. 62-66, PMID 7816848 , PMC 42817 (free full text) (review).
- ↑ C. Karavanich, J. Atema: Olfactory recognition of urine signals in dominance fights between male lobster, Homarus americanus. In: Behavior. Volume 135, Number 6, 1998, pp. 719-730.
- ^ A b D. K. Berger, MJ Butler: Octopuses influence den selection by juvenile Caribbean spiny lobster. In: Marine and Freshwater Research. Volume 52, 2001, pp. 1049-1053, doi: 10.1071 / MF01076
- ^ SG Ratchford, DB Eggleston: Temporal shift in the presence of a chemical cue contributes to a diel shift in sociality. In: Animal behavior. Volume 59, Number 4, April 2000, ISSN 0003-3472 , pp. 793-799, doi: 10.1006 / anbe.1999.1383 , PMID 10792934 .
- ↑ G. Nevitt, N. Pentcheff u. a .: Den selection by the spiny lobster Panulirus argus: testing attraction to conspecific odors in the field. In: Marine Ecology Progress Series. Volume 203, 2000, pp. 225-231, doi: 10.3354 / meps203225
- ↑ JC Barbato, PC Daniel: Chemosensory activation of an antennular grooming behavior in the spiny lobster, Panulirus argus, is tuned narrowly to L-glutamate. In: The Biological Bulletin. Volume 193, Number 2, 1997, pp. 107-115.
- ↑ PC Daniel, M. Shineman, M. Fischetti: Comparison of chemosensory activation of antennular grooming behavior in five species of decapods. In: Marine and Freshwater Research. Volume 52, 2001, pp. 1333-1337, doi: 10.1071 / MF01013
- ↑ a b J. Wroblewska, S. Whalley u. a .: Identification of chemosensory sensilla activating antennular grooming behavior in the Caribbean spiny lobster, Panulirus argus. In: Chemical senses. Volume 27, Number 9, November 2002, ISSN 0379-864X , pp. 769-778, PMID 12438202 .
- ↑ DW Dunham, KA Ciruna, HH Harvey: chemosensory role of antennules in the behavioral integration of feeding by the crayfish Cambarus bartonii. In: Journal of Crustacean Biology. Volume 17, 1997, pp. 27-32, doi : 10.1163 / 193724097X00052
- ^ AJ Horner, MJ Weissburg, CD Derby: Dual antennular chemosensory pathways can mediate orientation by Caribbean spiny lobsters in naturalistic flow conditions. In: The Journal of experimental biology. Volume 207, Pt 21October 2004, ISSN 0022-0949 , pp. 3785-3796, doi: 10.1242 / jeb.01200 , PMID 15371486 .
- ↑ KE Kraus-Epley, PA Moore: Bilateral and unilateral antennal lesions alter orientation abilities of the crayfish, Orconectes rusticus. In: Chem. Senses. Volume 27, number 1, 2002, pp. 49-55, doi: 10.1093 / chemse / 27.1.49
- ^ U. Grünert, BW Ache: Ultrastructure of the aesthetasc (olfactory) sensilla of the spiny lobster, Panulirus argus. In: Cell Tissue Research. Volume 251, Number 1, 1988, pp. 95-103, doi: 10.1007 / BF00215452
- ↑ CD Derby, HS Cate a. a .: Comparison of turnover in the olfactory organ of early juvenile stage and adult Caribbean spiny lobsters. In: Arthropod structure & development. Volume 31, Number 4, April 2003, ISSN 1873-5495 , pp. 297-311, doi : 10.1016 / S1467-8039 (02) 00050-6 , PMID 18088988 .
- ^ M. Schmidt, BW Ache: Antennular projections to the midbrain of the spiny lobster. II. Sensory innervation of the olfactory lobe. In: The Journal of comparative neurology. Volume 318, Number 3, April 1992, ISSN 0021-9967 , pp. 291-303, doi: 10.1002 / cne.903180306 , PMID 1583164 .
- ^ PB Reeder, BW Ache: Chemotaxis in the Florida lobster, Panulirus argus. In: Animal Behavior. Volume 28, Number 3, 1980, pp. 831-839, doi : 10.1016 / S0003-3472 (80) 80143-6
- ↑ CD Derby, P. Steullet u. a .: The sensory basis of feeding behavior in the Caribbean spiny lobster, Panulirus argus. ( Memento of the original from November 15, 2009 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. In: Marine Freshwater Research. Volume 52, 2001, pp. 1339-1350, doi: 10.1071 / MF01099
- ↑ P. Steullet, DR Kruetzfeldt u. a .: Dual antennular chemosensory pathways mediate odor-associative learning and odor discrimination in the Caribbean spiny lobster Panulirus argus. In: Journal of Experimental Biology . Volume 205, 2002, pp. 851-867, doi: 10.1242 / jeb.01200
- ^ A b R. Rajan, JP Clement, US Bhalla: Rats smell in stereo. In: Science. Volume 311, number 5761, February 2006, ISSN 1095-9203 , pp. 666-670, doi: 10.1126 / science.1122096 , PMID 16456082 .
- ^ DA Wilson, RM Sullivan: Respiratory airflow pattern at the rat's snout and an hypothesis regarding its role in olfaction. In: Physiology & behavior. Volume 66, Number 1, March 1999, ISSN 0031-9384 , pp. 41-44, PMID 10222471 .
- ^ WL Silver, DG Moulton: Chemosensitivity of rat nasal trigeminal receptors. In: Physiology & Behavior . Volume 28, Number 5, May 1982, ISSN 0031-9384 , pp. 927-931, PMID 7100294 .
- ↑ N. Uchida, ZF Mainen: Speed and accuracy of olfactory discrimination in the rat. In: Nature Neuroscience . Volume 6, Number 11, November 2003, ISSN 1097-6256 , pp. 1224-1229, doi: 10.1038 / nn1142 , PMID 14566341 .
- ↑ KC Catania: Epidermal sensory organs of moles, shrew moles, and desmans: a study of the family talpidae with comments on the function and evolution of Eimer's organ. In: Brain, behavior and evolution. Volume 56, Number 3, September 2000, ISSN 0006-8977 , pp. 146-174, doi: 10.1159 / 000047201 , PMID 11124516 .
- ^ KC Catania, JH Kaas: Organization of somatosensory cortex and distribution of corticospinal neurons in the eastern mole (Scalopus aquaticus). In: The Journal of Comparative Neurology . Volume 378, Number 3, February 1997, ISSN 0021-9967 , pp. 337-353, PMID 9034895 .
- ↑ jme / dpa: Spatial sense of smell: Second nostril guides moles to food. At: Spiegel Online from February 6, 2013
- ↑ K. Adam: Moles orientate themselves with a second nostril. At: focus.de on February 6, 2013
- ↑ Moles can smell stereo. At: scincexx.de on February 6, 2013
- ↑ A. Findeklee: Moles smell stereo. At: Spektrum.de on February 5, 2013
- ↑ DG Moulton: Olfaction in Mammals. In: American Zoologist. Volume 7, number 3, 1967, pp. 421-429, doi: 10.1093 / icb / 7.3.421
- ↑ Gerhard Aumüller , Gabriela Aust a. a .: Dual series anatomy. 2nd Edition. Georg Thieme Verlag, 2010, ISBN 978-3-13-152862-9 , p. 1039. limited preview in Google book search
- ↑ Thomas Hummel, Jens Reden, Johannes Frasnelli: Smell and Taste Perception. In: Joachim Funke , Peter A. Frensch (Hrsg.): Handbuch der Allgemeine Psychologie - Kognition. Hogrefe Verlag, 2006, ISBN 3-8409-1846-4 , pp. 152-156. limited preview in Google Book search
- ↑ a b A. M. Kleemann, J. Albrecht u. a .: Trigeminal perception is necessary to localize odors. In: Physiology & behavior. Volume 97, number 3-4, June 2009, ISSN 1873-507X , pp. 401-405, doi: 10.1016 / j.physbeh.2009.03.013 , PMID 19303891 .
- ^ Q. Chevy, E. Klingler: Odorless trigeminal stimulus CO2 triggers response in the olfactory cortex. In: The Journal of neuroscience. Volume 34, number 2, January 2014, ISSN 1529-2401 , pp. 341-342, doi: 10.1523 / JNEUROSCI.4466-13.2014 , PMID 24403134 .
- ^ F. Viana: Chemosensory properties of the trigeminal system. In: ACS Chemical Neuroscience . Volume 2, number 1, January 2011, ISSN 1948-7193 , pp. 38-50, doi: 10.1021 / cn100102c , PMID 22778855 , PMC 3369707 (free full text) (review).
- ↑ J. Frasnelli, J. Albrecht u. a .: Perception of specific trigeminal chemosensory agonists. In: Neuroscience. Volume 189, August 2011, ISSN 1873-7544 , pp. 377-383, doi: 10.1016 / j.neuroscience.2011.04.065 , PMID 21575683 , PMC 3150232 (free full text).
- ↑ M. Laska, H. Distel, R. Hudson: Trigeminal perception of odorant quality in congenitally anosmic subjects. In: Chemical Senses . Volume 22, Number 4, August 1997, ISSN 0379-864X , pp. 447-456, PMID 9279467 .
- ^ G. von Békésy : Olfactory analogue to directional hearing. In: Journal of applied physiology. Volume 19, May 1964, ISSN 0021-8987 , pp. 369-373, PMID 14173530 .
- ↑ JP Crimaldi, JR Koseff: High-resolution measurements of the structure of a turbulent plume. In: Experiments in Fluids. Volume 31, 2001, pp. 90-102.
- ↑ J. Frasnelli, G. Charbonneau et al. a .: Odor localization and sniffing. In: Chemical senses. Volume 34, Number 2, February 2009, ISSN 1464-3553 , pp. 139-144, doi: 10.1093 / chemse / bjn068 , PMID 19001464 .
- ↑ a b G. Kobal, S. Van Toller, T. Hummel: Is there directional smelling? In: Experientia . Volume 45, Number 2, February 1989, ISSN 0014-4754 , pp. 130-132, PMID 2493388 .
- ↑ T. Hummel, N. Roudnitzky et al. a .: Intranasal trigeminal function in children. In: Developmental medicine and child neurology. Volume 49, Number 11, November 2007, ISSN 0012-1622 , pp. 849-853, doi: 10.1111 / j.1469-8749.2007.00849.x , PMID 17979864 .
- ^ Therese Fark: Development of a taste test for school children between the ages of five and seven years. Dissertation . TU Dresden, 2012, pp. 19-20.








