Steroid 5- α -reductase
| Steroid 5- α -reductase 1 | ||
|---|---|---|
| Properties of human protein | ||
| Mass / length primary structure | 259 amino acids; 29.5 kDa | |
| Identifier | ||
| Gene names | SRD5A1 ; S5AR | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.3.99.5 , oxidoreductase | |
| Response type | Hydrogenation | |
| Substrate | 3-oxo-Δ 4.5 steroid | |
| Products | 3-Oxo-5 α -steroid | |
| Occurrence | ||
| Parent taxon | Euteleostomi | |
| Orthologue | ||
| human | mouse | |
| Entrez | 6715 | 78925 |
| Ensemble | ENSG00000145545; | ENSMUSG00000021594 |
| UniProt | P18405 | Q68FF9 |
| Refseq (mRNA) | NM_001047 | NM_175283 |
| Refseq (protein) | NP_001038 | NP_780492 |
| Gene locus | Chr 5: 6.69 - 6.72 Mb | Chr 13: 69.71 - 69.75 Mb |
| PubMed search | 6715 |
78925
|
| Steroid 5- α -reductase 2 | ||
|---|---|---|
| Properties of human protein | ||
| Mass / length primary structure | 254 amino acids; 28.4 kDa | |
| Identifier | ||
| Gene name | SRD5A2 | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.3.99.5 , oxidoreductase | |
| Response type | Hydrogenation | |
| Substrate | 3-oxo-Δ 4.5 steroid | |
| Products | 3-Oxo-5 α -steroid | |
| Occurrence | ||
| Parent taxon | Euteleostomi | |
| Orthologue | ||
| human | mouse | |
| Entrez | 6716 | 94224 |
| Ensemble | ENSG00000049319 | ENSMUSG00000038541 |
| UniProt | P31213 | Q99N99 |
| Refseq (mRNA) | NM_000348 | NM_053188 |
| Refseq (protein) | NP_000339 | NP_444418 |
| Gene locus | Chr 2: 31.6 - 31.66 Mb | Chr 17: 74.37 - 74.4 Mb |
| PubMed search | 6716 |
94224
|
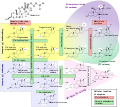
Steroid-5 α -reductase (SRD5) (also testosterone 5 α -reductase or 5 α -reductase for short ) is the name for three well-known enzymes that are found in vertebrates and are very similar (so-called isozymes ). These isozymes enable, among other things, the conversion of the sex hormone testosterone into the biologically more effective dihydrotestosterone and are therefore necessary for the effects of this hormone. The absence of in particular the second isozyme (SRD5A2) results in men to malformation of the urethra , known as hypospadias , and a haplotype of SRD5A2, with consequent increased 5 α -reductase activity was in women with an increased risk of polycystic ovary syndrome associated. On the other hand, the inhibition of the enzymes has beneficial effects in diseases of the prostate and hair loss .
structure
Exist on the three enzyme isoforms , which as a 5 α -reductase type I, type II and type III are referred to and their genes (SRD5A1, SRD5A2 and SRD5A3) on different chromosomes ( 5 , 2 and 4 ) lie. The primary structure of the two hydrophobic membrane proteins (5α-reductase type I and type II) consists of 259 and 254 amino acids , with a molecular mass of 29,459 and 28,393 Da .
Biological function
Metabolism of steroid hormones e
Steroid 5- α -reductase is a NADPH -dependent enzyme from the group of oxidoreductases which irreversible, the reduction of 3-oxo-Δ 4,5 - steroids catalysis to give the corresponding 3-oxo-5α-compounds. By far the most important reaction is the conversion of the sex hormone testosterone into the biologically more effective dihydrotestosterone (DHT).
physiology
The isoenzymes of 5 α -reductase are produced to varying degrees in many organs and tissues in the human body:
- Type I is mainly found in the brain , epididymis, and skin . It plays an important role in the biosynthesis of allopregnanolone, wherein he progesterone in 5 α converts -Dihydroprogesteron, which is converted via a further step ultimately allopregnanolone (ALLO). It is, like tetrahydrodeoxycorticosterone (THDOC), a positive allosteric modulator of GABA A receptors , which is the same place of action of euphoric and anti-anxiety drugs , such as. B. benzodiazepines have.
- Type II is mainly found in the muscles , liver , kidneys , prostate and is only produced to a very small extent in the skin . In benign prostatic hyperplasia (BPH) there is a strong increase in this type.
- Type III, which was discovered later and is probably the most important, is primarily produced in the brain , in the mammary glands , cervix , skin , ovaries , testes , prostate , pancreas , spleen , kidneys , heart , stomach and liver . Here, too, there is a slight increase in BPH . Type III is also of great importance for glycosylation : it converts polyprenol to dolichol . The gene of this type, SRD5A3, is mutated in patients with some form of congenital disorder of glycosylation (CDG).
An increased production of the enzyme also takes place in polycystic ovaries in women, which is why it may play a role in polycystic ovarian syndrome .
The lack or even absence of type II in the male fetus can lead to hypospadias and intersexuality .
An inhibition of these three isoenzymes of 5 α -reductase leads to a reduced production of dihydrotestosterone and allopregnanolone, but to a slight increase in the testosterone level, and in some cases also of estradiol . The development of gynecomastia , depression, erectile dysfunction and loss of libido are some of the possible side effects of 5α-reductase inhibition, which can persist even after discontinuation. This type of persistent side effect is known as post-finasteride syndrome .
pharmacology
5 α- reductase inhibitors are drugs that are used, for example, in the therapy of benign prostatic hyperplasia (BPH) and in androgenic alopecia . Examples are finasteride and dutasteride . Finasteride blocks the function of type II and type III isoenzymes, while dutasteride inhibits all three isoenzymes.
These inhibitors are derived from the structure of testosterone. It has not yet been possible to examine the structures of the isoenzymes by means of X-ray structure analysis. The only information available is the primary structure of the proteins derived from the cDNA.
See also
literature
- S. Andersson et al .: Deletion of steroid 5 alpha-reductase 2 gene in male pseudohermaphroditism. In Nature . Volume 354, number 6349, November 1991, pp. 159-161, doi: 10.1038 / 354159a0 , PMID 1944596 , PMC 4451825 (free full text).
- Julianne Imperato-McGinley et al .: Steroid 5alpha-reductase deficiency in man: an inherited form of male pseudohermaphroditism. In Science . Volume 186, Number 4170, December 1974, pp. 1213-1215, PMID 4432067 .
- S. Andersson et al .: Structural and biochemical properties of cloned and expressed human and rat steroid 5 alpha-reductases. In: Proceedings of the National Academy of Sciences . Volume 87, Number 10, May 1990, pp. 3640-3644, PMID 2339109 , PMC 53958 (free full text).
- DW Russell et al .: Steroid 5 alpha-reductase: two genes / two enzymes. In Annu Rev Biochem . Volume 63, 1994, pp. 25-61, doi: 10.1146 / annurev.bi.63.070194.000325 , PMID 7979239 (review).
- T. Ishikawa et al .: Aromatase-independent testosterone conversion into estrogenic steroids is inhibited by a 5 alpha-reductase inhibitor. In: The Journal of steroid biochemistry and molecular biology. Volume 98, Number 2-3, February 2006, pp. 133-138, doi: 10.1016 / j.jsbmb.2005.09.004 , PMID 16386416 .
- A. Iranmanesh et al .: Combined inhibition of types I and II 5 alpha-reductase selectively augments the basal (nonpulsatile) mode of testosterone secretion in young men. In: The Journal of clinical endocrinology and metabolism. Volume 90, Number 7, July 2005, pp. 4232-4237, doi: 10.1210 / jc.2004-2262 , PMID 15811930 .
- MH Choi et al .: Biochemical roles of testosterone and epitestosterone to 5 alpha-reductase as indicators of male-pattern baldness. In J Invest Dermatol. Volume 116, Number 1, January 2001, pp. 57-61, doi: 10.1046 / j.1523-1747.2001.00188.x , PMID 11168798 .
Web links
- Bruce E Wilson: 5-Alpha-Reductase Deficiency
Individual evidence
- ↑ UniProt P31213
- ↑ UniProt P18405 .
- ↑ a b A. Godoy, E. Kawinski et al. a .: 5α-reductase type 3 expression in human benign and malignant tissues: a comparative analysis during prostate cancer progression. In: The Prostate . Volume 71, number 10, 2011, pp. 1033-1046. doi: 10.1002 / pros.21318 . PMID 21557268 .
- ↑ a b c K. Yamana, F. Labrie u. a .: Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride . In: Hormone Molecular Biology and Clinical Investigation , Volume 2, No. 3, August 1, 2010.
- ↑ a b M. Streiber: Hybrid inhibitors of human 5α-reductase: A new concept for the inhibition of the 5α-reductase isoenzymes type I and type II . Dissertation, Saarbrücken University, 2006, urn : nbn: de: bsz: 291-scidok-10733 .
- ^ BG Gunn, AR Brown, JJ Lambert, D Belelli: Neurosteroids and GABA (A) Receptor Interactions: A Focus on Stress . In: Frontiers in Neuroscience . 5, 2011, p. 131. doi : 10.3389 / fnins.2011.00131 . PMID 22164129 . PMC 3230140 (free full text).
- ^ V. Cantagrel, DJ Lefeber a. a .: SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. In: Cell . Volume 142, Number 2, July 2010, pp. 203-217, ISSN 1097-4172 . doi: 10.1016 / j.cell.2010.06.001 . PMID 20637498 . PMC 2940322 (free full text).
- ↑ a b SRD5AR2. In: Online Mendelian Inheritance in Man . (English).
- ↑ Pseudovaginal Perineoscrotal Hypospadia. In: Online Mendelian Inheritance in Man . (English).
- ↑ C. Trombetta, G. Mazzon, G. Liguori, G. Ollandini, S. Cauci, G. Toffoli, E. Erika: Clinical analysis in young patient with persistent sexual dysfunctions after finasteride assumption to prevent male pattern hair loss. In: European Urology Supplement. Volume 11, No. 1, February 2012, pp. E698-e698a, doi: 10.1016 / S1569-9056 (12) 60695-2 .
- ^ B Römer, P Gass: Finasteride-induced depression: new insights into possible pathomechanisms . In: Journal of Cosmetic Dermatology . Volume 9, No. 4, December 2010, pp. 331-2. doi : 10.1111 / j.1473-2165.2010.00533.x . PMID 21122055 .
- ^ I. Goldstein: An old problem with a new cause-5 alpha reductase inhibitors and persistent sexual dysfunction. In: The Journal of Sexual Medicine . Volume 8, Number 7, July 2011, pp. 1829-1831, ISSN 1743-6109 . doi: 10.1111 / j.1743-6109.2011.02368.x . PMID 21762384 .
- ↑ Propecia leaflet (PDF; 210 kB), Merck (American package insert )
- ↑ MS Irwig, S. Kolukula: Persistent sexual side effects of finasteride for male pattern hair loss. In: The journal of sexual medicine. Volume 8, Number 6, June 2011, pp. 1747-1753, ISSN 1743-6109 . doi: 10.1111 / j.1743-6109.2011.02255.x . PMID 21418145 .
- ↑ AM Traish, J. Hassani et al. a .: Adverse side effects of 5α-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. In: The journal of sexual medicine. Volume 8, Number 3, March 2011, pp. 872-884, ISSN 1743-6109 . doi: 10.1111 / j.1743-6109.2010.02157.x . PMID 21176115 . (Review).