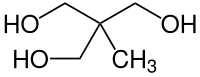
Trimethylolethane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Trimethylolethane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 12 O 3 | |||||||||||||||
| Brief description |
white crystal powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 120.15 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point | ||||||||||||||||
| boiling point | ||||||||||||||||
| solubility |
soluble in water, methanol , ethanol and acetone , very soluble in acetic acid , insoluble in diethyl ether and benzene |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Trimethylolethane is a trivalent polyalcohol ( polyol ) with a neopentyl structure, in which three hydroxymethyl groups and one methyl group are positioned on a quaternary carbon atom. Like other spherical molecules, such as B. Adamantane or the parent compound neopentane shows 1,1,1-tris (hydroxymethyl) ethane plastically crystalline properties, d. H. a low long-range orientation order in the solid state, which is accompanied by a soft, waxy consistency. TME esters are characterized by their high stability towards temperature, light and hydrolysis.
Manufacturing
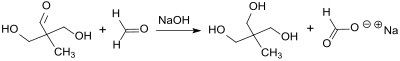
In the reaction of propionaldehyde with formaldehyde in a ratio of 1: 3 in the presence of quicklime (calcium oxide), 1,1,1-tris (hydroxymethyl) ethane, known as pentaglycerine, was obtained and characterized as early as 1893.
In 1901 it was recognized that an aldol reaction initially takes place in the presence of potassium carbonate and the aldol obtained can be reduced to pentaglycerol with aluminum amalgam .
The double aldol addition of formaldehyde in aqueous solution (formalin) to propionaldehyde initially leads to 2,2-dihydroxymethylpropionaldehyde in a smooth reaction and can be kept at this stage with the appropriate procedure.
The subsequent cross Cannizzaro reaction with excess formaldehyde in the presence of sodium hydroxide gives trimethylolethane in yields of approx. 90%.
Because of the high solubility in water and the high boiling point of 1,1,1-tris (hydroxymethyl) ethane, it cannot simply be separated from the sodium formate , which is produced in stoichiometric amounts . After removing the excess formaldehyde and most of the water, TME can be extracted with isopropanol or methyl isobutyl ketone MIBK, isolated by crystallization and highly purified by sublimation.
Since aldol addition and Cannizzaro reaction take place simultaneously at elevated temperatures and in alkaline conditions, the overall reaction can also be carried out continuously with yields> 90%.
A modern process variant avoids the Cannizzaro reaction with the stoichiometric (and usually undesirable) by-product sodium formate and uses solid heterogeneous catalysts in the aqueous medium for the first stage of the aldol addition ( anion exchanger with amino groups ) and for the second stage of the hydrogenation of the aldol addition product ( Nickel - chromium or copper - chromium catalyst). After the excess formaldehyde has been removed by distillation, the catalytic hydrogenation gives a quantitative yield of trimethylolethane.
properties
Trimethylolethane is a solid that is obtained as a white, hygroscopic crystal powder or when recrystallising from alcohols as “white, comb-like needles” or as prismatic needles. The triol dissolves very well in acetic acid and alcohols, as well as in water, with solubilities of 140 g / l at 25 ° C, 60 g / l at 20 ° C and 40 g / l at 25 ° C being given. TME is practically insoluble in non-polar solvents. The compound tastes sweet like other polyols.
Because of the lack of hydrogen atoms in the vicinity of the hydroxyl groups , the molecule has a low tendency towards thermal elimination. The spatial shielding of the hydroxyl groups against radical attack increases their stability against heat and light and the stability z. B. the TME ester versus hydrolysis.
Applications
Trimethylolethane forms with carboxylic acids under acidic catalysis or by transesterification simple carboxylic acid esters in the presence of titanium catalysts, such as. B. tetrabutyl orthotitanate or tin catalysts, such as. B. dibutyltin oxide, the corresponding mono-, di- and triesters and their mixtures.
Esters of trimethylolethane with longer branched carboxylic acids are used as stabilizers and as plasticizers for plastics, while those with fatty acids are used as emulsifiers .
Reaction products with ethylene oxide EO or propylene oxide PO are used as polyester and polyurethane components. TME esters combine high temperature resistance with low temperature viscosity and are suitable for demanding applications such as B. synthetic lubricants for internal combustion engines and aircraft engines.
The compact TME molecule gives alkyd resins and polyester resins for high quality paint applications improved weather resistance, as well as higher hardness and scratch resistance. The nitration of 1,1,1-tris (hydroxymethyl) ethane with oleum - nitric acid mixtures or nitrating acid produces trinitrate, which is used as an explosive and as a propellant component for solid rockets.
Trimethylolethane acts as a tridentate O donor ligand for copper - catalyzed cross - coupling reactions of aryl iodides with amides , thiols, and phenols in good to excellent yields.
Trimethylolethane is suitable in a mixture with water and possibly other polar substances, such as. B. Sulfolane or urea , as so-called phase change materials (English. Phase change materials, PCM ) with phase transition temperatures between 0 ° C and 25 ° C and high thermal storage density. Such mixtures can be used as passive heat storage or as cold storage media in the building sector.
literature
- Peter Wehrle, Marcus Morawietz, Stefan Lundmark, Kent Sörensen, Esko Karvinen, Juha Letonen: Alcohols, Polyhydric . In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2008, ISBN 978-3-527-30673-2 , doi : 10.1002 / 14356007.a01_305.pub2 .
Individual evidence
- ↑ a b c d e Entry on Trimethylolethane at TCI Europe, accessed on April 30, 2018.
- ^ A b David R. Lide: CRC Handbook of Chemistry and Physics, 86th Edition . CRC Press, Boca Raton, FL, USA 2005, ISBN 0-8493-0486-5 , pp. 3-294 .
- ↑ Data sheet 1,1,1-Tris (hydroxymethyl) ethane from Sigma-Aldrich , accessed on April 30, 2018 ( PDF ).
- ↑ a b c TRIMETR TME (Trimethylolethane) Product Data, Technical Data Sheet. (PDF; 325 kB) In: [email protected]. GEO Specialty Chemicals, 2017, accessed April 30, 2018 .
- ↑ a b c G.J. Laemmle, JG Milligan, WJ Peppel: Trimethylolethane from propionaldehyde and formaldehyde . In: Ind. Eng. Chem. Band 52 , no. 1 , 1960, p. 33-36 , doi : 10.1021 / ie.50601a032 .
- ↑ a b R. Pummerer, H. Hahn, F. John, H. Kehlen: About the photochemical structure of branched carbon chains from ether and formaldehyde . In: Chem. Ber. tape 75 , no. 7 , 1942, pp. 867-881 , doi : 10.1002 / cber.19420750719 .
- ^ A b William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, Boca Raton, FL, USA 2016, ISBN 978-1-4987-5429-3 , pp. 3-310 .
- ↑ There is not yet a harmonized classification for this substance . A labeling of ethylidynetrimethanol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on October 30, 2018, is reproduced from a self-classification by the distributor .
- ↑ A Complete Guide to TRIMETR Brand of Trimethylolethane. (PDF; 448 kB) In: [email protected]. GEO Specialty Chemicals, 1999, accessed April 30, 2018 .
- ↑ a b H. Hosaeus: XXXVI. About the penta-glycerine (methyl-trimethylol-methane) . In: Liebigs Ann. Chem. Band 276 , no. 1 , 1893, p. 75-79 , doi : 10.1002 / jlac.18932760108 .
- ↑ H. Koch, T. Zerner: About the condensation of propionic and formaldehyde . In: monthly Chem. Band 22 , no. 5 , 1901, pp. 443-459 , doi : 10.1007 / BF01524089 .
- ↑ Patent US4247485 : Process for the preparation of 2,2-dimethylolalkanals. Registered on March 19, 1979 , published on January 27, 1981 , applicant: Bayer AG, inventor: O. Immel, H.-H. Schwarz, H. Quast.
- ↑ Patent US2790837 : Continuous production of trimethylolethane. Filed June 8, 1954 , published April 30, 1957 , applicant: Celanese Corp., inventor: MO Robeson.
- ↑ T. Salmi, V. Serra-Holm, T.-K. Rantakylä, P. Mäki-Arvela, LP Lindfors: Development of clean technology for the production of triols . In: Green Chem. Band 1 , 1999, p. 283-288 , doi : 10.1039 / A907691C .
- ↑ Y.-J. Chen, H.-H. Chen: 1,1,1-Tris (hydroxymethyl) ethane as a new, efficient, and versatile tripod ligand for copper-catalyzed cross-coupling reactions of aryl iodides with amides, thiols, and phenols . In: Org. Lett. tape 8 , no. 24 , 2006, pp. 5609-5612 , doi : 10.1021 / ol062339h .
- ↑ H. Kakiuchi, M. Yabe, M. Yamazaki: A study of trimethylolethane hydrate as a phase change material . In: J. Chem. Eng. Jpn. tape 36 , no. 7 , 2003, p. 788-793 , doi : 10.1252 / jcej.36.788 .
- ↑ Patent EP1113065A1 : Heat-storage material composition. Applied on March 3, 1999 , published on July 4, 2001 , applicant: Mitsubishi Chemical Corp., inventor: M. Yabe, H. Kakiuchi, M. Yamazaki.