1,2-diaminocyclohexane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structural formulas of ( R , R ) -1,2-diaminocyclohexane (top left), ( S , S ) -1,2-diaminocyclohexane (top right) and meso -1,2-diaminocyclohexane | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 1,2-diaminocyclohexane | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 14 N 2 | ||||||||||||||||||
| Brief description |
colorless liquid with an amine-like odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 114.19 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.95 g cm −3 |
||||||||||||||||||
| boiling point |
183 ° C |
||||||||||||||||||
| Vapor pressure |
11.5 h Pa (70 ° C) |
||||||||||||||||||
| solubility |
soluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
1,2-Diaminocyclohexane is a chemical compound . It consists of a cyclohexane ring as the basic structure, to which two amino groups are bonded to adjacent carbon atoms and thus belongs to the group of diamines .
Isomerism
1,2-Diaminocyclohexane contains two stereogenic centers, consequently there are three stereoisomers: ( R , R ) -1,2-diaminocyclohexane and the mirror image of ( S , S ) -1,2-diaminocyclohexane and meso -1,2-diaminocyclohexane . The 1: 1 mixture of ( R , R ) - and the enantiomeric ( S , S ) form is the racemate trans -1,2-diaminocyclohexane. Meso -1,2-diaminocyclohexane is sometimes also called cis -1,2-diaminocyclohexane.
presentation
1,2-Diaminocyclohexane can be produced by Curtius degradation of 1,2-cyclohexanedicarboxylic acid. If the trans -1,2-cyclohexanedicarboxylic acid is used as the starting material, the amino groups in the product are also arranged in the trans position. Starting from cis- 1,2-cyclohexanedicarboxylic acid ( meso- 1,2-cyclohexanedicarboxylic acid), cis- 1,2-diaminocyclohexane ( meso- 1,2-diaminocyclohexane) is obtained analogously . The resolution of trans -1,2-diaminocyclohexane [1: 1 mixture of ( R , R ) -1,2-diaminocyclohexane and ( S , S ) -1,2-diaminocyclohexane] into its enantiomers can be carried out via diastereomeric salts manage the treatment with enantiomerically pure tartaric acid .
The cis -1,2-diaminocyclohexane (in the form of its sulfate salt) can be obtained from the isomer mixture by reaction with nickel chloride and subsequent treatment with sulfuric acid and ethanol.
properties
It is a colorless compound that is liquid at room temperature and boils at 183 ° C. The rotation of the pure substance at 55 ° C and a wavelength of 589 nm is −36 ° [( R , R ) -enantiomer].
use
Through condensation reactions with α, β- diketones , 1,2-diaminocyclohexane can be used for the synthesis of pyrazines . With elimination of two water molecules are so initially diimine obtained, which then pyrazines oxidized can be.
By condensation with activated carboxylic acids ( Steglich esterification ) or carboxylic acid chlorides can amides are prepared. This reaction is used, for example, to synthesize the Trost ligand .

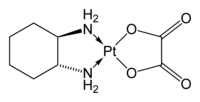
In complex chemistry , 1,2-diaminocyclohexane can be used as a bidentate chelating ligand . For example, oxaliplatinous compounds that are used as cytostatics are platinum complexes of 1,2-diaminocyclohexane.
In addition, 1,2-diaminocyclohexane condenses with two equivalents of a salicylaldehyde derivative to form a salen ligand . These tetradentate chelating ligands form with cobalt (II) complexes , as oxygen transporters , for example, in the Jacobsen epoxidation used.
Individual evidence
- ↑ a b c d e data sheet 1,2-diaminocyclohexane (PDF) from Merck , accessed on January 18, 2011.
- ↑ a b Entry on cyclohex-1,2-ylenediamine in the GESTIS substance database of the IFA , accessed on February 8, 2018(JavaScript required) .
- ^ Heinrich Otto Wieland , Otto Schlichting, Werner von Langsdorff: Hoppe-Seyler's Journal for Physiological Chemistry , Vol. 161 (1926), p. 76, ISSN 0018-4888 .
- ^ A b F. M. Jaeger, Lucas Bijkerk: About complex salts of trivalent cobalt, chromium and rhodium with racemic and optically active trans-cyclohexane-1,2-diamines . In: Journal for inorganic and general chemistry , Vol. 233 (1937), pp. 97-139. ISSN 0044-2313 , doi: 10.1002 / zaac.19372330202 .
- ↑ Prisca K. Eckert, Verena Schill, Carsten Strohmann: Synthesis of cis-TMCDA: Optimization and characterization of a key intermediate . In: Inorganica Chimica Acta . 2011, doi : 10.1016 / j.ica.2011.06.008 .
- ↑ Bernhard Johannes Lüssem: Palladium-catalyzed enantioselective synthesis of allylic thiocarboxylates and palladium-catalyzed deracemization of allylic carbonates (PDF; 7.8 MB) . Dissertation, RWTH Aachen 2004.
- ↑ Konstantin P. Bryliakov, Evgenij P. Talsi: European Journal of Organic Chemistry , Vol. 11 (2008), pp. 3369-3376, ISSN 1099-0690 .