2,5-diaminohydroquinone dihydrochloride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,5-diaminohydroquinone dihydrochloride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 10 Cl 2 N 2 O 2 | ||||||||||||||||||
| Brief description |
gray to brown powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 213.06 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point | |||||||||||||||||||
| solubility |
soluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2,5-diaminohydroquinone dihydrochloride DAHQ is a symmetrical dihydroxybenzene and phenylenediamine with both hydroxy and amino groups in the para position to one another, which is usually present as an oxidation- stable dihydrochloride salt. DAHQ is the raw material for organic electrode materials for rechargeable batteries , for organic semiconductors and for MOFs .
DAHQ reacts with carboxylic acids to form trans -benzobisoxazoles and with dicarboxylic acids such as B. terephthalic acid or terephthalic acid dichloride , for the (non-commercial) trans form of the high-performance plastic poly (p-phenylene-2,6-benzobisoxazole) PBO ( trans -PBO).
Occurrence and representation
The starting material 2,5-dimethoxy-1,4-benzoquinone DMBQ, which is easily accessible from hydroquinone or 1,4- benzoquinone, is reacted in methanolic solution under pressure with ammonia to give 2,5-diamino-1,4-benzoquinone and then with tin (II) chloride dihydrate SnCl 2 · 2H 2 O reduced in the presence of hydrochloric acid to 2,5-diamino-1,4-benzenediol dihydrochloride.
1,4-Benzoquinone can also be converted to DMBQ with Raney nickel in methanol, which reacts with ammonia in methanol to form 2,5-diamino-1,4-benzoquinone and can also be reduced to DAHQ with sodium dithionite in aqueous solution.
Alternatively, chloranil is reacted in 2-methoxyethyl acetate with aqueous ammonium hydroxide solution to give the intermediate 2,5-diamino-3,6-dichloro-1,4-benzoquinone (95% yield), which in water on a heterogeneous palladium contact gives the end product DAHQ dihydrochloride is hydrogenated (yield> 85%).
When the two process steps were revised under optimized process conditions, yields of 84% in the first and 95% in the second stage with purities> 96% were recently found.
properties
During the synthesis, 2,5-diaminohydroquinone dihydrochloride is obtained as a white to brown water-soluble powder, which only melts at temperatures above 300 ° C. In its properties, it is very similar to the positionally isomeric 4,6-diaminoresorcinol dihydrochloride DAR, which is also described as very sensitive to oxidation. Like DAR, DAHQ can also be recrystallized to colorless needles from hot, dilute hydrochloric acid with the addition of activated carbon and the reducing agent tin (II) chloride as an oxidation inhibitor.
Applications
In many applications, the purity of the oxidation-sensitive diaminodihydroxybenzenes such as 2,5-diaminohydroquinone is critical. Quantitative carbonylation with ring closure to oxazolones produces stable, protected intermediates from which the corresponding diaminodihydroxybenzene can be completely recovered with concentrated hydrochloric acid .
DAHQ as a building block for conductive materials
When 2,5-diaminohydroquinone dihydrochloride is dissolved in water, the base triethylamine is added and the mixture is stirred in air, a dark purple precipitate of the oxidation product 2,5-diamino-1,4-benzoquinone DABQ, which is being investigated as an electrode material for organic lithium batteries , is quickly formed .
Electrically conductive organometallic networks MOFs are in practically quantitative yield from 2,5-dihydroxy-1,4-benzoquinone DHBQ - from 2,5-diaminohydroquinone via its oxidation product DABQ and its hydrolysis
- and tetrabutylammonium bromide TBAB, NBu 4 Br and nickel (II) acetate Ni (OAc) 2 accessible.
With thiophene-2-carbaldehyde and oxygen with 4-methoxy- TEMPO as the oxidation catalyst, a bis-thiophene-substituted benzobisoxazole is formed, which has been characterized as a model substance with an extended conjugated π-electron system for organic electroluminescent materials .
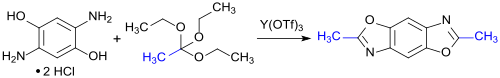
A similar approach - condensation of 2,5-diaminohydroquinone dihydrochloride with orthoesters , such as. B. triethyl orthoacetate (R = CH 3 ), in the presence of the Lewis acid yttrium triflate - is pursued in the synthesis of low molecular weight linear fused benzobisoxazole ring systems, which have been investigated for their suitability as organic semiconductors because of their good electron transport properties (n-type) .
DAHQ as a monomer for trans -PBO
As the positionally 4,6-diaminoresorcinol dihydrochloride can be 2,5-diaminohydroquinone dihydrochloride with aromatic dicarboxylic acids, preferably terephthalic acid or carboxylic acid chloride Terephthalolychlorid to trans - poly (p-phenylene-2,6-benzobisoxazole) trans -PbO polycondense ,
which, however, was processed much less intensively and described in the literature. The main area of application for the polymer PBO was originally fibers for fabrics in ballistic protective vests . The theoretical modulus of elasticity for fibers made from trans -PBO is given as 707 GPa , only slightly less than the extremely high value for cis -PBO fibers (730 GPa), but significantly higher than for the high-performance plastics trans - polybenzimidazole PBI with 640 or UHMWPE (ultra high molecular weight polyethylene) with 362 GPa. Recently there has been anecdotal evidence of a higher linearity, as well as better mechanical properties and photoelectric behavior of trans -PBO fibers compared to their cis counterparts.
According to NIST findings , the tensile strength of protective vests made of PBO fibers decreases by 30% when used under the influence of moisture and temperature, which led to the decertification of PBO fibers in protective vests and the rapidly decreasing interest in PBO as a research object.
Individual evidence
- ↑ a b data sheet 2,5-diamino-1,4-dihydroxybenzenedihydrochloride from AlfaAesar, accessed on June 25, 2020 ( PDF )(JavaScript required) .
- ↑ a b c data sheet 2,5-diamino-hydroquinone-dihydrochloride from Sigma-Aldrich , accessed on June 25, 2020 ( PDF ).
- ↑ a b Safety Data Sheet: 2,5-Diaminohydroquinone Dihydrochloride. Fujifilm, September 20, 2019, accessed June 28, 2020 .
- ↑ a b L. Sieuw, A.Jouhara, E. Quarez, C. Auger, J.-F. Gohy, P. Poizot, A. Vlad: A H-bond stabilized quinone electrode material for Li-organic batteries: the strength of weak bonds . In: Chem. Sci. tape 10 , 2019, pp. 418-426 , doi : 10.1039 / c8sc02995d .
- ↑ a b J.F. Mike, AJ Makowski, M. Jeffries-EL: An efficient synthesis of 2,6-disubstituted benzobisoxazoles: New building blocks for organic semiconductors . In: Org. Lett. tape 10 , no. 21 , 2008, p. 4915-4918 , doi : 10.1021 / ol802011y .
- ↑ a b K.V. Nielsen, L. Zhang, Q. Zhang, TL Liu: A strategic high yield synthesis of 2,5-dihydroxy-1,4-benzoquinone based MOFs . In: Inorg. Chem. Band 58 , no. 16 , 2019, pp. 10756-10760 , doi : 10.1021 / acs.inorgchem.9b00903 .
- ↑ Patent US2743286 : Process for preparing alkoxyquinone compounds. Filed March 12, 1952 , published April 24, 1956 , Applicant: Eastman Kodak Co., Inventor: GF Rodgers.
- ↑ Patent JP2007070300A : Process for producing 2,5-diamino-1,4-benzoquinone and 2,5-diamino-1,4-benzenediol, and salt thereof. Applied on September 8, 2005 , published on March 22, 2007 , applicant: Teijin Ltd., inventor: M. Jokai, H. Kuwabara.
- ^ VK Ahluwalia, P. Bhagat, R. Aggarwal, R. Chandra: Intermediates for Organic Synthesis . IK International Publishing House Pvt. Ltd., New Delhi 2005, ISBN 978-81-88237-33-3 , pp. 23 .
- ↑ Patent US4806688 : Method for producing 3,6-bis (carboethoxyamino) -2,5-diaziridinyl-1,4-benzoquinone. Applied on August 29, 1980 , published June 29, 1982 , applicant: United States of America, inventor: SJ Backlund, RE Olsen.
- ↑ Patent WO198706930 : Preparation of diamino-and dialkylaminobenzenediols. Applied on May 12, 1987 , published November 19, 1987 , Applicant: The Dow Chemical Co., Inventor: MN Inbasekaran, RM Strom.
- ↑ M. Inbasekaran, R. Strom: A convenient synthesis of 2,5-diamino-1,4-benzene diol . In: OPPI Briefs . tape 23 , no. 4 , 1991, pp. 447-450 , doi : 10.1080 / 00304949109458235 .
- ↑ a b B. Dong, J. Zhang, C. Wu, N. Jin, D. Zhao: Synthesis of trans-PBO AA type monomeric 2,5-diamino-hydroquinone hydrochloride . In: Chemical Industry and Engineering Progress . tape 6 , 2019 ( com.cn ).
- ↑ Patent US5892116 : Process for producing 4,6-diaminoresorcinols. Filed October 8, 1997 , published April 6, 1999 , Applicants: Daiwa Kasei Industry Co., Ltd., Inventors: J. Kawachi, H. Matsubara, Y. Nakahara, Y. Watanabe.
- ↑ T. Mizuno, T. Nakai, M. Mihara, T. Ito: Facile sulfur-assisted carbonylation of diaminoresorcinol with carbon monoxide . In: Heteroatom Chem. Volume 23 , no. 1 , 2012, p. 111-116 , doi : 10.1002 / hc.20746 .
- ↑ L.-Q. Chai, Y.-L. Zhang, J.-F. Tong, G. Liu: Synthesis, Crystal Structure and Fluorescence Behavior of 2,6-Di (thiophen-2-yl) -benzo [1,2-d: 4,5-d '] bisoxazole . In: Z. Naturforsch. 68b, 2013, p. 239–244 , doi : 10.5560 / ZNB.2013-2301 .
- ↑ X.-S. Yi, S. Du, L. Zhang (Eds.): Composite Materials Engineering, Volume 1: Fundamentals of Composite Materials . Chemical Industry Press, Beijing 2006, ISBN 978-981-10-5695-6 , pp. 116 .
- ↑ J. Chin, A. Forster, C. Clerici, L. Sung, M. Oudina, K. Rice: Temperature and humidity aging of poly (p-phenylene-2,6-benzobisoxazole) fibers: Chemical and physical characterization . In: Polym. Degradation. Stable. tape 92 , 2007, p. 1234-1246 , doi : 10.1016 / j.polymdegradstab.2007.03.030 .