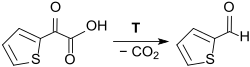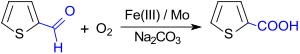Thiophene-2-carbaldehyde
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
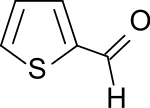
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Thiophene-2-carbaldehyde | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 5 H 4 OS | ||||||||||||||||||
| Brief description |
clear, colorless to dark yellow liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 112.15 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.2127 g cm −3 at 21 ° C |
||||||||||||||||||
| boiling point | |||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.5920 (25 ° C, 589 nm) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Thiophene-2-carbaldehyde is a five-membered heteroaromatic with one sulfur atom and an adjacent aldehyde group in the 2-position. The 2-formylthiophene is the starting material for several important drugs .
Occurrence and representation
2-thiophenecarboxaldehyde occurs naturally in camembert (0.4 mg / kg) and in coffee (up to 1.8 mg / kg).
As early as 1885, the formation of 2-thiophenaldehyde during the thermal decomposition of 2-thienylglyoxylic acid (from 2-acetylthiophene by alkaline oxidation with potassium permanganate ) with the elimination of CO 2 was reported.
For the chemical synthesis of 2-thienylaldehyde, there are two elaborated laboratory instructions in Organic Syntheses , based on 2-chloromethylthiophene (which is very irritating to the skin and tears and tends to decompose explosively), which is reacted with hexamethylenetetramine to form the corresponding ammonium salt and then with steam with yields decomposed by 48–53% to the aldehyde.
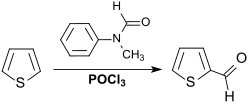
A more recent specification describes the Vilsmeier reaction (an electrophilic aromatic substitution ) on the electron-rich aromatic thiophene with N-methylformanilide and phosphorus oxychloride and decomposition of the salt formed with water in 71-74% yield.
The product yield reaches 87% if the reaction temperatures are kept at 55-65 ° C. and the aldehyde is isolated by steam distillation .
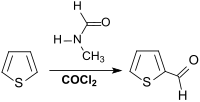
The patent literature describes the reaction of thiophene with dimethylformamide DMF and phosgene , in which 2-thiophenaldehyde is obtained in 98% yield and the expensive disposal of phosphate-containing wastewater is avoided.
Electron-rich aromatics such as thiophene react in the presence of Lewis acids, such as. B. tin (IV) chloride SnCl 4 or titanium (IV) chloride TiCl 4 with (dichloromethyl) methyl ether as the formylating agent in a reaction named after Alfred Rieche as Rieche formylation to give thiophene-2-carbaldehyde.
The α-methoxythienyl chloride formed as an intermediate splits off HCl during the aqueous-acidic work-up and forms 2-formylthiophene in a 90% yield.
properties
Thiophene-2-carbaldehyde is similar to the benzene derivative benzaldehyde and is described in the older literature as a “colorless, water-white oil with a pleasant odor reminiscent of bitter almond oil”. Due to the presence of traces of odor-intensive decomposition products, the aldehyde is usually characterized as a pungent, malodorous colorless liquid that turns yellow to brown in air and should therefore be stored under nitrogen or with hydroquinone as an oxidation inhibitor.
Applications
2-thiophenaldehyde as a building block for APIs
Teniposide is a semi-synthetic podophyllotoxin glycoside in which thiophene-2-carbaldehyde is bound to the glucose residue in a semi - acetal manner in order to reduce the cytotoxic side effects of the cytostatic agent .
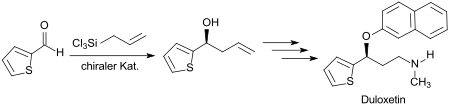
A synthetic route for the antidepressant duloxetine starts from 2-thiophenaldehyde, which is allylated with allyltrichlorosilane with> 97% ee in the first stage in the presence of a strong Lewis base and an enantioselective catalyst acting as a bipyridine- di- N-oxide .
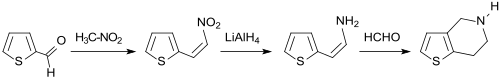
A critical building block for the platelet aggregation inhibitors ticlopidine and clopidogrel is a dinuclear heterocyclic ring system in which the five-membered thiophene with a six-membered tetrahydropyridine ring forms the 4,5,6,7-tetrahydrothieno [3,2-c] pyridine is fused . For this purpose, thiophene-2-aldehyde is converted in a Henry reaction with nitromethane in alkaline conditions to form the corresponding nitroaldol, which upon acidification - analogous to β-nitrostyrene - eliminates water and forms 2- (2-nitrovinyl) thiophene. The α, β-unsaturated nitroalkene reacts with sodium borohydride NaBH 4 to form the amine 2- (2-aminoethyl) thiophene and this in a Pictet-Spengler reaction with formaldehyde to form the formimino compound, which cyclizes to the target product on shaking with dilute hydrochloric acid.
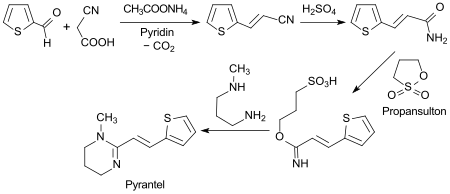
A standard synthesis of the anthelmintic pyrantel starts with an aldol condensation of thiophene-2-carbaldehyde with cyanoacetic acid to give 3- (2-thienyl) acrylonitrile.
Synthesis variants of the less common active pharmaceutical ingredients azosemide, eprosartan and tenylidone are also based on thiophene-2-carbaldehyde.
Simple derivatives of 2-thiophene aldehyde
2-Hydroxymethylthiophene is formed in the Cannizzaro reaction of 2-thiophenaldehyde with formaldehyde with 59% yield or in the reduction with sodium borohydride in water (96%).
Oxidation of 2-thiophenaldehyde with oxygen on an iron (III) molybdenum contact gives thiophene-2-carboxylic acid in high yield (92%) under mild conditions
The Cu (I) thiophene-2-carboxylate ( CuTC ) formed from thiophene-2-carboxylic acid and copper (I) oxide Cu 2 O is suitable - in combination with tetrakis (triphenylphosphine) palladium (0)) - as a catalyst for the linkage of aryl halides ( Ullmann reaction ). CuTC also co-catalyzes the cross-coupling of aryl iodides or alkenyl iodides and boronic acids and represents an alternative to the Suzuki coupling .
literature
- Salo Gronowitz, Anna-Britta Hörnfeldt: Thiophenes. , Elsevier Ltd., Kidlington, UK 2004, ISBN 0-12-303953-3 , p. 71.
- Jürgen Engel, Axel Kleemann , Bernhard Krischer, Dietmar Reichert: Pharmaceutical Substances: Syntheses, Patents and Applications of the most relevant APIs, 5th Edition, Thieme, Stuttgart, 2009, ISBN 978-3-1317-9275-4 .
Individual evidence
- ↑ Entry on 2-thiophenecarboxaldehyde (stabilized with HQ) at TCI Europe, accessed on June 30, 2020.
- ↑ a b c d e f g data sheet Thiophene-2-carboxaldehyde from AlfaAesar, accessed on June 30, 2020 ( PDF )(JavaScript required) .
- ^ A b c Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Oxford, UK 2015, ISBN 978-0-323-28659-6 , pp. 64 .
- ↑ Scientific Opinion on Flavoring Group Evaluation 21, Revision 2 (FGE.21Rev2). In: Thiazoles, thiophene, thiazoline and thienyl derivatives from chemical group 29. Miscellaneous substances from chemical group 30. EFSA European Food Safety Authority, October 12, 2011, accessed July 1, 2020 .
- ↑ a b A. Peter: Studies on the β-Acetotiënon and its derivatives . In: Ber. German Chem. Ges. Volume 18 , no. 1 , 1885, p. 537-542 , doi : 10.1002 / cber.188501801117 .
- ↑ A. Biedermann: About thiophenaldehyde and the compound of the thiophene series corresponding to benzyl alcohol . In: Ber. German Chem. Ges. Volume 19 , no. 1 , 1886, p. 636-640 , doi : 10.1002 / cber.188601901144 .
- ^ KB Wiberg, HF McShane: 2-Chloromethylhiophene In: Organic Syntheses . 29, 1949, p. 31, doi : 10.15227 / orgsyn.029.0031 ; Coll. Vol. 3, 1955, p. 197 ( PDF ).
- ↑ KB Wiberg: 2-Thiophenaldehyde In: Organic Syntheses . {{{Volume}}}, {{{Volume}}}, p. {{{Pages}}}, doi : 10.15227 / orgsyn.000.0005 ; Coll. Vol. 3, 1955, p. 811 ( PDF ).
- ^ WJ King, FF Nord: Preparation of thiophene-2-aldehyde and some substituted thiophene aldehydes . In: J. Org. Chem. Band 13 , no. 5 , 1948, pp. 635-640 , doi : 10.1021 / jo01163a003 .
- ↑ AW Weston, RJ Michaels, Jr .: 2-Thenaldehyde In: Organic Syntheses . 31, 1951, p. 108, doi : 10.15227 / orgsyn.031.0108 ; Coll. Vol. 4, 1963, p. 915 ( PDF ).
- ↑ Patent US2741622 : Preparation of thiophene-2-aldehyde. Applied December 3, 1951 , published April 10, 1956 , Applicant: Monsanto Chemical Co., Inventor: DJ Brockmeyer, FC Meyer.
- ↑ A. Rieche, H. Gross, E. Höft: About α-halogen ethers. IV. Syntheses of aromatic aldehydes with dichloromethyl alkyl ethers . In: Chem. Ber. tape 93 , no. 1 , 1960, p. 88-94 , doi : 10.1002 / cber.19600930115 .
- ↑ H. Stähelin, A. von Wartburg: From podophyllotoxin glucoside to etoposide . In: Prog. Drug Res. Band 33 , 1989, pp. 169-266 , doi : 10.1007 / 978-3-0348-9146_8 .
- ↑ P. Motloch, I. Valterová, M. Kotora: Enantioselective allylation of thiophene-2-carbaldehyde: Formal total synthesis of Duloxetine . In: Adv. Synth. Catal. tape 356 , no. 1 , 2014, p. 199-204 , doi : 10.1002 / adsc.201300849 .
- ↑ Patent EP342118A1 : 2- (2-nitrovinyl) thiophene reduction and synthesis of thieno (3,2-c) pyridine derivatives. Applied on May 9, 1989 , published November 15, 1989 , applicant: Sanofi, inventor: E. Lodewijk, HN Khatri.
- ↑ Jürgen Engel, Axel Kleemann, Bernhard Krischer, Dietmar Reichert: Pharmaceutical Substances: Syntheses, Patents and Applications of the most relevant APIs, 5th Edition . Thieme, Stuttgart 2009, ISBN 978-3-13-179275-4 , p. 1160 .
- ↑ FW Dunn, K. Dittmer: A microbial synthesis of 2-thiophenecarbinol . In: J. Am. Chem. Soc. tape 68 , no. 12 , 1946, pp. 2561-2562 , doi : 10.1021 / ja01216a040 .
- ↑ H. Yu, S. Ru, G. Dai, Y. Zhai, H. Lin, S. Han, Y. Wei: An efficient iron (III) -catalyzed aerobic oxidation of aldehydes in water for the green preparation of acrboxylic acids . In: Angew. Chem. Int. Ed. tape 56 , no. 14 , 2017, p. 3867–3871 , doi : 10.1002 / anie.201612225 .
- ↑ A. Innitzer: Copper (I) Thiophenes-2-carboxylate . In: Synlett . tape 15 , 2005, pp. 2405-2406 , doi : 10.1055 / s-2005-872681 .
- ↑ C. Savarin, LS Liebeskind: Nonbasic, room temperature, palladium-catalyzed coupling of aryl and alkenyl iodides with boronic acids mediated by copper (I) thiophene-2-carboxylate (CuTC) . In: Org. Lett. tape 3 , no. 14 , 2001, p. 2149-2152 , doi : 10.1021 / ol010060p .