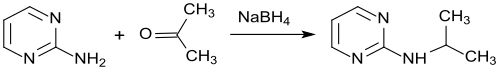2-aminopyrimidine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
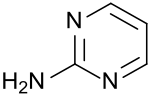
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-aminopyrimidine | ||||||||||||||||||
| other names |
Pyrimidin-2-amine |
||||||||||||||||||
| Molecular formula | C 4 H 5 N 3 | ||||||||||||||||||
| Brief description |
white to light yellow crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 95.10 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
122-126 ° C |
||||||||||||||||||
| boiling point |
158-160 ° C |
||||||||||||||||||
| solubility |
soluble in water, and in methanol |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2-aminopyrimidine is a nitrogen-containing heterocycle that is derived from pyrimidine , a 1,3-diazine, and carries a primary amino group . The pyrimidin-2-amine is found as a parent compound in agrochemicals such. B. fungicides and insecticides and is a molecular building block for the sulfonamide sulfadiazine, which in combination with a dihydrofolate reductase - inhibitor for the treatment of toxoplasmosis , usually but when the silver salt silver sulfadiazine is used for wound treatment.
Occurrence and representation
When the (unstable and therefore not isolated) 3-oxopropanoic acid (formylacetic acid) formed from malic acid with 20% oleum is reacted with guanidine salts , isocytosine is formed in 69% yield.
The 4- hydroxyl group of isocytosine present in tautomeric equilibrium can be exchanged with phosphorus oxychloride in a strongly acidic medium for a chlorine atom in 93% yield to give 2-amino-4-chloropyrimidine. The chlorine atom in this compound is easily removed with zinc dust in ammoniacal solution to form 2-aminopyrimidine (yield 90%).
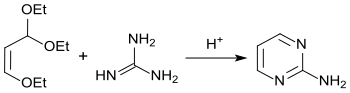
An alternative synthetic route starts from β-ethoxyacroleindiethylacetal, which reacts in the presence of strong acids with guanidine hydrochloride with a yield of 53% to form pyrimidin-2-amine.
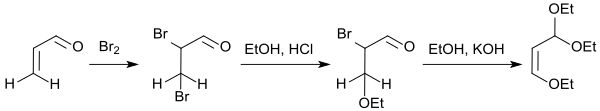
The elaborate preparation of ethoxyacroleindiethylacetal from acrolein by bromination to dibromopropanal and its conversion with ethanol and hydrogen chloride to form β-ethoxybromopropanal diethyl acetal (64% yield) and subsequent dehydrobromination with potassium hydroxide in ethanol to give the actual reactant β-ethoxyacrolein diethyl acetal (80% yield)
and the overall low yields make this synthetic route uneconomical.
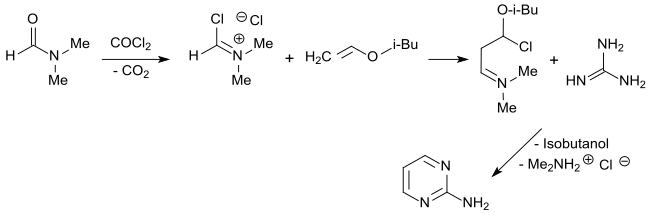
An alternative that is also interesting on a larger scale is the route via the malondialdehyde derivative formed from the Vilsmeier salt (from dimethylformamide and phosgene ) with isobutyl vinyl ether , which forms 2-aminopyrimidine with guanidine hydrochloride in 97% yield.
properties
2-aminopyrimidine is a white crystalline solid that dissolves in water and polar solvents.
Applications
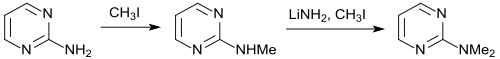
The methylation of 2-aminopyrimidine to 2-methylaminopyrimidine succeeds with methyl iodide in ethanol in 90% yield. After activation with lithium amide in benzene , a second methyl group can also be introduced using methyl iodide , albeit with a low yield (31%). The synthesis of 2-dimethylaminopyrimidine succeeds with 88% yield by reaction of 2-chloropyrimidine and dimethylamine .
2-Isopropylaminopyrimidine can be obtained from 2-aminopyrimidine by reaction with acetone and subsequent hydrogenation with sodium borohydride in 60% pure yield.
The compound was tested as a phosphate salt for efficacy against neuropathies , but was not pursued further because of hepatotoxicity .
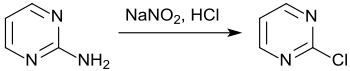
From pyrimidin-2-amine, 2-chloropyrimidine can be obtained in modest yields (26–27%) by diazotization in concentrated hydrochloric acid
and can be easily aminated to 2-arylaminopyrimidines with arylamines .
2-chloropyrimidine is also used in the last stage of the synthesis of the dopamine agonist piribedil .
The so-called 2-aminopyrimidine fungicides effective against powdery mildew, such as As the now no longer registered in Germany Products ethirimol , dimethirimol and bupirimate , as well as the insecticides pirimiphos-methyl , or Primidophos Pyrimidate, is the 2-aminopyrimidine-structure basis.
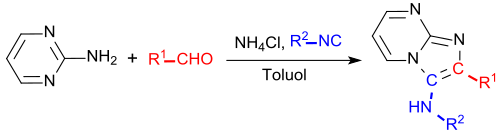
In a multicomponent reaction with three reactants - an aldehyde , an isonitrile and the amine 2-aminopyrimidine - a one-pot reaction according to Ivar Karl Ugi results in moderate to good yields (around 50%) 3-amino-imidazo [1,2-a] pyrimidine:
However, according to more recent studies, the reaction produces a mixture of the 2- and 3-amino compounds.
Another revision of this reaction with toluene as the non-polar solvent and sequential supply of the reactants and addition of ammonium chloride as promoter leads to a uniform 3-amino product in yields of around 60%.
The most important use of 2-aminopyrimidine is as an amine molecular building block for the sulfonamide sulfadiazine, which in combination with trimethoprim or pyrimethamine is the agent of choice for the treatment of toxoplasmosis in animals and humans.
In the penultimate (n-1) step of the preparation of sulfadiazine, 4-acetamidobenzene sulfochloride is converted with pyridin-2-amine to form the acetyl-protected sulfadiazine, which is then deacetylated to the end product.
Individual evidence
- ↑ Entry on 2-aminopyrimidines at TCI Europe, accessed on November 10, 2017.
- ↑ a b data sheet 2-pyrimidinamine from Sigma-Aldrich , accessed on November 10, 2017 ( PDF ).
- ↑ Data sheet 2-aminopyrimidine for synthesis (PDF) from Merck , accessed on November 10, 2017.
- ↑ a b c data sheet 2-aminopyrimidines from AlfaAesar, accessed on November 10, 2017 ( PDF )(JavaScript required) .
- ↑ Entry on 2-aminopyrimidines at TCI Europe, accessed on November 10, 2017.
- ↑ a b Alan Wood, Compendium of Pesticide Common Names, http://www.alanwood.net/pesticides/index.html
- ↑ Patent US2224836 : 2-aminopyrimidines. Filed April 26, 1940 , published December 10, 1940 , Applicant: American Cyanamid Co., Inventor: RO Roblin, Jr., JP English.
- ↑ WT Caldwell, HB Kime: A new synthesis of isocytosine . In: J. Am. Chem. Soc. tape 62 , no. 9 , 1940, p. 2365 , doi : 10.1021 / ja01866a028 .
- ↑ Patent US2425248 : Production of 2-aminopyrimidine. Filed February 3, 1945 , published August 5, 1947 , Applicant: American Cyanamid Co., Inventor: E. Kuh, TW Clapper.
- ↑ Patent US2344707 : Process for producing 2-aminopyrimidine. Applied on September 19, 1942 , published March 21, 1944 , applicant: American Cyanamid Co., inventor: E. Kuh.
- ↑ RW Price, A. Moos: A new synthesis of 2-aminopyrimidine . In: J. Am. Chem. Soc. tape 67 , no. 2 , 1945, p. 207–208 , doi : 10.1021 / ja01218a018 .
- ↑ Patent US2375735 : Preparation of pyrimidines. Filed November 8, 1941 , published May 8, 1945 , Applicant: Lederle Laboratories, Inc., Inventor: AM Moos, RW Price.
- ↑ E. Fischer, G. Giebe: Representation of the acetals . In: Ber. German Chem. Ges. Volume 30 , no. 3 , 1897, p. 3053-3059 , doi : 10.1002 / cber.189703003121 .
- ↑ L. Claisen: On the knowledge of propargylaldehyde and phenylpropargylaldehyde . In: Ber. German Chem. Ges. Volume 36 , no. 3 , 1903, pp. 3664-3673 , doi : 10.1002 / cber.19030360168 .
- ↑ Patent US3974159 : Manufacture of derivatives of malondialdehyde. Applied on April 28, 1975 , published on August 10, 1976 , Applicant: BASF AG, Inventors: M. Decker, W. Schoenleben, H. Toussaint, H. Hoffmann.
- ↑ CG Overberger, IC Kogon: Monomer synthesis. Methylation of 2-aminopyrimidine . In: J. Am. Chem. Soc. tape 76 , no. 4 , 1954, pp. 1065-1068 , doi : 10.1021 / ja01633a040 .
- ↑ Patent US4266057 : Process for the preparation of 2-isopropylamino pyrimidine. Applied on June 11, 1979 , published May 5, 1981 , applicant: Expansia, inventor: CG Demosthene, CR Aspisi.
- ↑ IC Kogon, R. Minin, CG Overberger: 2-Chloropyrimidine In: Organic Syntheses . 35, 1955, p. 34, doi : 10.15227 / orgsyn.035.0034 ; Coll. Vol. 4, 1963, p. 336 ( PDF ).
- ↑ K. Walsh, HF Sneddon, CJ Moody: Amination of heteroaryl chlorides: Palladium catalysis or SNAr in green solvents? In: Chemsuschem . tape 6 , no. 8 , 2013, p. 1455-1460 , doi : 10.1002 / cssc.201300239 .
- ↑ K. Groebke, L. Weber, F. Mehlin: Synthesis of Imidazo [1,2-a] annulated Pyridines, Pyrazines and Pyrimidines by a Novel Three-Component Condensation . In: Synlett . tape 1998 , no. 6 , 1998, pp. 661-663 , doi : 10.1055 / s-1998-1721 .
- ↑ GS Mandair, M. Light, A. Russell, M. Hursthouse, M. Bradley: Re-evaluation of the outcome of a multiple component reaction - 2- and 3-amino-imidazo [1,2-a] pyrimidines? In: Tetrahedron Lett. tape 43 , no. 23 , 2002, p. 4267-4269 , doi : 10.1016 / S-0040-4039 (02) 00709-8 .
- ↑ VZ Parchinsky, O. Shuvalova, O. Ushakova, DV Kravchenko, M. Krasavin: Multi-component reactions between 2-aminopyrimidine, aldehydes and isonitriles: the use of a nonpolar solvent suppresses formation of multiple products . In: Tetrahedron Lett. tape 47 , no. 6 , 2006, p. 947-951 , doi : 10.1016 / j.tetlet.2005.11.152 .
- ^ RO Roblin, Jr., JH Williams, PS Winnek, JP English: Chemotherapy. II. Some sulfanilamide heterocycles . In: J. Am. Chem. Soc. tape 62 , no. 8 , 1940, p. 2002–2005 , doi : 10.1021 / ja01865a027 .