2-hydroxy-4-methylthiobutyric acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||||
| Simplified structural formula without stereochemistry - 1: 1 mixture of enantiomers | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-hydroxy-4- (methylthio) butyric acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 5 H 10 O 3 S | ||||||||||||||||||
| Brief description |
yellow to brown liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 150.2 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.21-1.23 g · cm −3 at 25 ° C |
||||||||||||||||||
| Melting point |
10 ° C |
||||||||||||||||||
| boiling point |
120 ° C |
||||||||||||||||||
| Vapor pressure |
16 mmHg (25 ° C) |
||||||||||||||||||
| solubility |
completely soluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2-Hydroxy-4-methylthiobutyric acid is the racemic hydroxy analog of the naturally occurring sulfur- containing essential proteinogenic amino acid L - methionine . The functional hydroxycarboxylic acid HMTBA can be absorbed from the feed by animals and converted into L- methionine, which promotes protein formation and is used to increase the milk protein content in ruminants and for faster growth of other animals, e.g. B. poultry and fish is used.
Occurrence and representation
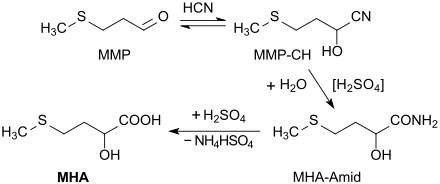
The considerable importance of methionine substitutes in livestock nutrition has led to the investigation of several enzymatic and chemical synthesis routes, of which the route, first patented in 1956, via methyl mercaptopropionaldehyde MMP (by Michael addition of methyl mercaptan to acrolein ) has established itself as the standard process on an industrial scale.
In a cyanohydrin synthesis , hydrocyanic acid HCN at pH 7.5 adds almost quantitatively to MMP to form 2-hydroxy-4-methylthiobutyronitrile (also known as MMP cyanohydrin or MMP-CH). The cyano group is first diluted with 65% sulfuric acid at 50 ° C. to form the amide group and then with water and hydrolyzed at 90 ° C. to give the carboxyl group . The reactions to the MHA amide and further to the acid MHA also proceed with a very high yield to> 98% 2-hydroxy-4-methylthiobutyric acid , the approx. 15% MHA ester (so-called MHA oligomers ) - predominantly the MHA dimer - contain.
The reaction product is a yellow to brown viscous solution of MHA in water, which contains equimolar amounts of ammonium bisulfate NH 4 HSO 4 , which reacts with ammonia or ammonium hydroxide to form ammonium sulfate (NH 4 ) 2 SO 4 . With gentle removal of the water under vacuum, a suspension with crystalline ammonium sulfate is formed, from which by extraction, e.g. B. with acetone , the organic product can be separated. The product freed from the extractant is a yellow-brown liquid, the viscosity of which depends heavily on the content of oligomers. For further purification, the crude hydroxycarboxylic acid can be distilled as gently as possible (to avoid the formation of oligomers which reduce the nutritional value of HMB) in a vacuum, whereby colorless and storage-stable MHA is obtained.
In industrial practice, MHA synthesis is carried out in a continuous process.
The ammonium sulphate obtained in equimolar amounts in cyanohydrin hydrolysis - just as in the hydrolysis of acetone cyanohydrin to the end product methacrylic acid methyl ester MMA - represents a considerable waste water or disposal problem in other process variants. The pure ammonium sulphate can either be used as a fertilizer or thermally broken down to ammonia and sulfuric acid which can both be fed back into the process.
properties
2-Hydroxy-4-methylthiobutyric acid is a yellow to brown, viscous liquid with a pungent sulfur-like odor, which is strongly acidic (pH <1 in 100% form). Commercially available HMTBA is an aqueous, flowable concentrate adjusted to a content of 88%, which contains less than 17% oligomers and can still easily be sprayed onto granulated or pelleted animal feed. If MHA is stored for a long time, oligomeric intermolecular esters are formed, the concentration of which can rise to approx. 50%.
Applications
After absorption in the animal organism, DL -2-hydroxy-4-methylthiobutyric acid is converted to α- keto acid by oxidation and transamination to the amino acid L - methionine (Met). Met plays an important role in cell metabolism as an antioxidant and a source of sulfur , e.g. B. for the amino acid cysteine or the tripeptide glutathione , or as a methyl group donor, e.g. B. for choline , however, is not sufficiently available in many feed sources for farm animals.
While L- methionine and the racemate DL- Met is absorbed by active transport in the stomach and duodenum , MHA can be absorbed both by active transport and by passive diffusion and essentially metabolized in the liver into L- Met.
Findings from extensive studies indicate that 2-hydroxy-4-methylthiobutyric acid is a useful source for protein synthesis in non-ruminants and fish, but has a lower biological potency than DL- methionine. In ruminants (cattle, sheep, goats), MHA is broken down more slowly in the rumen than DL -Met.
The 88% MHA is said to be around 20% cheaper than DL-methionine on a comparative basis. Due to its acidity, it saves feed additives of organic acids, e.g. B. lactic acid , and inhibits the growth of fungi and pathogenic bacteria in the feed. The administration of HMTBA leads to a lower nitrogen excretion of the animals fed with it.
The calcium salt of 2-hydroxy-4-methylthiobutyric acid is a solid with effects comparable to MHA.
The isopropyl ester of HMB, known as HMBi, is available in liquid (95% HMBi) and solid (57% HMBi) form and is broken down more slowly and to a lesser extent in the rumen of cattle than the acid MHA (and as DL -Met). 50% of the ester is metabolized in the rumen and 50% absorbed through the rumen wall and converted to L- Met in the liver . When administered to dairy cows, HMBi is said to increase protein and fat content.
Manufacturer and trade name
2-Hydroxy-4-methylthiobutyric acid is sold by Adisseo , a subsidiary of ChemChina , under the brand name Rhodimet® AT 88, by Novus International Inc., a joint venture of Mitsui & Co., Ltd. and Nippon Sōda , manufactured under the brand name ALIMET® and by Sumitomo Chemical under the brand name SUMIMET ™ -L and marketed mainly as a feed supplement for pigs, poultry and animals in aquaculture (fish, shrimp).
The isopropyl ester of 2-hydrox-4-methylthiobutyric acid is sold by Adisseo as MetaSmart® for dairy cows.
The calcium salt of 2-hydrox-4-methylthiobutyric acid is used by Adisseo as ADRY + ® and Novus as MFP® for animal feed, and by Evonik under the REXIM® brand name as DL-alpha-hydroxymethionine, calcium salt - together with L- methionine and others Amino acids - offered to treat chronic kidney failure .
Individual evidence
- ↑ a b c d e f g h Safety Data Sheet Rhodimet AT 88 (liquid HMB). (PDF; 87 KB) Adisseo Canada Inc., August 8, 2018, accessed on May 15, 2020 (English).
- ↑ a b c GPS Safety Summary ALIMET®. (PDF; 87 KB) Novus International, Inc., December 1, 2015, accessed on May 15, 2020 .
- ↑ Patent US2745745 : Poultry feed. Applied October 30, 1952 , published May 15, 1956 , Applicant: Monsanto Chemical Co., Inventor: ES Blake, RJ Wineman.
- ↑ Patent US4912257 : Process for the preparation of aqueous solutions of 2-hydroxy-4-methylthio butyric acid. Applied on February 13, 1989 , published March 27, 1990 , applicant: Sociedad de Desarrollo Tecnico Industrial, inventor: JA Hernandez, LR Moreno.
- ↑ Patent WO199605173 : Process for the production of 2-hydroxy-4-methylthiobutyric acid (MHA) and its use as an animal feed supplement. Registered on August 12, 1994 , published on February 22, 1996 , applicant: Degussa AG, inventor: H. Suchsland, V. Häfner.
- ↑ Patent EP1444166B1 : Chemical and thermal decomposition of ammonium sulfate to ammonia and sulfuric acid. Filed September 26, 2002 , published September 5, 2007 , applicant: Silica Tech ANS, inventor: T. Hansen.
- ^ RL Levine, J. Moskovitz, ER Stadtman: Oxidation of methionine in proteins: roles in antioxidant defense and cellular regulation . In: IUBMB Life . tape 50 , no. 4-5 , 2000, pp. 301-307 , doi : 10.1080 / 713803735 .
- ↑ a b EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP): Safety and efficacy of hydroxy analogue of methionine and its calcium salt (ADRY + ®) for all animal species . In: EFSA Journal . tape 16 , no. 3 , 2018, p. 5198 , doi : 10.2903 / j.efsa.2018.5198 .
- ↑ MetaSmart®, The unique isopropyl methionine analog for ruminants. (PDF; 5.5 MB) Adisseo, 2015, accessed on May 15, 2020 (English).
- ↑ ZH Chen, GA Broderick, ND Luchini, BK Sloan, E. Devillard: Effect of feeding different sources of rumen-protected methionine on milk production and N-utilization in lactating dairy cows . In: J. Dairy Sci. tape 94 , no. 4 , 2011, p. 1978–1988 , doi : 10.3168 / jds.2010-3578 .