Chlorhexidine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
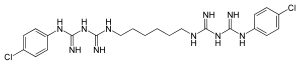
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Chlorhexidine | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 22 H 30 Cl 2 N 10 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 505.45 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
134-136 ° C |
||||||||||||||||||
| pK s value |
10.78 (25 ° C) |
||||||||||||||||||
| solubility |
poor in water (800 mg l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Chlorhexidine ( CHX ) is an antiseptic that is mainly used in dentistry . Due to its unspecific antibacterial effect, chlorhexidine is used both as a mouthwash solution and as a varnish applied to the teeth, which releases the active ingredient over a longer period of time. Outside of dentistry, it is used in topical wound care as a disinfectant on plasters, wound healing ointments and in powders, for example in navel care for newborns .
properties
The molecule is doubly positively charged in neutral aqueous solution . It is commercially available as chloride or acetate ; for medical applications, chlorhexidine di gluconate is mostly used [chlorhexidine bis ( D -gluconate)]. The molecule is mirror-symmetrical and contains two benzene rings . Chlorhexidine is poorly soluble in water , but good in organic solvents such as dichloromethane . In contrast, the gluconate is readily soluble in water.
It is inactivated by sodium lauryl sulfate and triclosan , both of which are often found in toothpaste . For this reason, a toothpaste free of sodium lauryl sulfate and triclosan should be used during oral treatment with chlorhexidine. Alternatively, there should be an interval of at least one hour between brushing your teeth and using chlorhexidine.
Antibacterial effect
Chlorhexidine penetrates the bacterial cell membrane and changes it. At high concentrations it has a bactericidal effect through structural damage to the membrane; at low concentrations it leads to the loss of small molecules and the precipitation of cytoplasmic proteins, which has a bacteriostatic effect. The highest activity is shown against gram-positive cocci such as B. Streptococcus mutans , one of the main causes of dental caries , lower levels against gram-positive and -negative rods . Acid-fast rods (causing tuberculosis (Mycobacterium tuberculosis), leprosy (Mycobacterium leprae) and diphtheria (Corynebacterium diphtheriae)) and spores are resistant. A moderate effect can be observed with enveloped viruses , non- enveloped viruses are not affected.
Chlorhexidine adheres to the teeth and oral mucosa for a long time without penetrating the body through the mucous membranes, which speaks for a long-lasting effect. If ingested, it is metabolized in extremely small amounts .
use
Due to its unspecific antibacterial effect, chlorhexidine is used both as a mouthwash solution and as a varnish applied to the teeth that releases the active ingredient over a longer period of time (around three to four months). There are also chlorhexidine sprays, gels and chips. In dentistry, chlorhexidine is used in concentrations of 0.03 to 2% (as a chip up to 36%) for the following indications:
- preoperatively (before oral surgery) in order to achieve a relative freedom from bacteria and to prevent bacteremia
- postoperatively to counteract the impaired oral hygiene in the operating area
- for rinsing root canals as part of an endodontic treatment (2%)
- as first aid or supportive as part of periodontal therapy:
- in the case of bacterial gingivitis or periodontitis in terms of all-round disinfection
- for ulcerating-necrotizing courses of gingivitis ( NUG ) or periodontitis ( NUP )
- as CHX gel (1.5%) and CHX chip (30–36%) for insertion into periodontal pockets
- to inhibit the formation of new dental plaques ( tooth decay prevention )
- for bad breath (halitosis)
- with dry mouth
- as part of "full-mouth disinfection", for brushing the back of the tongue (1%) and for rinsing (0.2%)
- as a dental spray (1.5%) for disinfecting toothbrushes and dentures
The clinical efficacy of chlorhexidine as a component of a mouthwash solution is evident from a number of studies that have been summarized in review articles. In direct comparison with other antibacterially active compounds, chlorhexidine proves to be superior, which is attributed to the active ingredient's good mucosal adhesion.
Mouth rinses containing chlorhexidine often contain 6-7% ethanol to increase their effectiveness and shelf life and to preserve them despite possible health risks such as cancer ; however, there are also effective mouthwashes containing chlorhexidine that do not contain ethanol.
Outside of dentistry, it is used in topical wound healing care as a disinfectant , such as B. anoint on plasters, wound healing and in powders. Chlorhexidine powder is used in the navel care of newborns. In a study published in 2009, navel care with chlorhexidine powder was found to be superior to dry care. The results showed that "umbilical care with chlorhexidine powder (1%) significantly reduced the navel-associated adverse events compared to dry care."
Chlorhexidine is also used to disinfect the skin and has been shown to be superior to PVP iodine in a study (in combination with a 70% 2-propanol solution) . Together with mupirocin , it is also used to eliminate MRSA in the nasal atrium.
According to the Federal Institute for Drugs and Medical Devices (BfArM), the product information has been supplemented with the following risk information since October 2014 :
"According to analysis of case reports and publications, the risk of chemical burns in newborns after the use of alcoholic as well as aqueous chlorhexidine solutions for skin disinfection before invasive interventions seems to be increased."
Side effects
The use of chlorhexidine causes few, mostly completely reversible side effects after prolonged use:
- Disorder of taste ( dysgeusia )
- Brownish deposits on teeth, gums and tongue (to varying degrees depending on the product)
- Desquamation of the epithelial cell layer (rare)
- Anaphylactic reaction
For long-term home use, it is recommended to alternate chlorhexidine and a non-chlorhexidine mouthwash solution at weekly intervals in order to reduce the side effects mentioned. The brownish deposits on teeth and tongue stem from the fact that bacterial proteins are denatured when the bacterial cell membranes are destroyed and disulfide functions are reduced to thiol functions , which form dark-colored complexes with the iron (III) ions of saliva . Other discolorations could result from the fact that monosaccharides such as glucose and fructose dissolved in the saliva react with the amine functions of bacterial proteins ( Maillard reaction ).
The original assumption that the extent of the discoloration is proportional to the effectiveness of products containing chlorhexidine has to be questioned for several reasons. As long as chlorhexidine can store itself in the bacterial membrane and the substantivity of the chlorhexidine is not impaired, products containing chlorhexidine should not lose their effectiveness. Attempts to prevent the brownish deposits using reducing agents such as ascorbic acid , which react with iron (III) ions, and using nucleophiles such as sulfite ions, which react with glucose and fructose , were then also successful.
Clinical studies with periodontitis patients show that postoperative, seven-day, adjuvant treatment with chlorhexidine-containing (0.2%), ethanol-free mouthwashes is not impaired by the addition of ascorbic acid and sulfite , while the extent of discoloration is significantly reduced by this addition (used commercially as the so-called "Anti Discoloration System" ).
A clinical study with healthy volunteers, which did not examine the status of the gums but rather various plaque parameters , came to the conclusion that there was a difference in effectiveness in favor of the conventional formulation. The authors attributed this difference not only to the lack of ethanol , but also to the fact that the ascorbic acid and sulfite in the ethanol- free mouthwash solution could prevent the desired adhesion of chlorhexidine to teeth and gums. But why the uncharged ascorbic acid or the negatively charged ascorbate or the negatively charged sulfite should prevent the adhesion of the doubly positively charged chlorhexidine to teeth and gums, the authors do not explain. The conceivable combination in the sense of electrostatic attraction ( Coulomb's law ) between negatively charged sulfite or ascorbate and positively charged chlorhexidine to possibly insoluble chlorhexidine sulfite or chlorhexidine ascorbate does not take place. The substantivity of chlorhexidine should therefore be preserved by adding sulfite or ascorbic acid.
The apparent contradiction between the gum status study and the plaque study is probably due to the fact that different study parameters were chosen. Although plaque is a necessary prerequisite for inflammation of the gums ( gingivitis ), the plaque study with healthy volunteers does not, strictly speaking, allow any conclusions to be drawn about the effect of a product on the gum status of periodontal disease patients.
The potential ototoxicity of skin disinfectants, including chlorhexidine, has been known since 1971. In the meantime, repeated animal studies have confirmed that chlorhexidine - when it enters the middle ear , e.g. B. in the presence of an eardrum perforation - can cause permanent hearing damage.
Trade names
Antebor (CH), anti-infect dental spray (D, A), Bepanthen Antiseptische Wundcreme (D), Bepanthen Plus (CH), Cervitec Gel / liquid / Plus (FL), Chlorhexamed (D, A, CH), ChloSite Gel ( EU, CH), Cidegol C (D), Collu-Blache (CH), Collunosol (CH), Corsodyl (A, CH), Curasept ADS (EU, CH), Cristalmina (ES), Dentohexin (CH), DermaPlast ( CH), Dynexan Proaktiv (D), Eludril (CH), Hexal-Solution (D), Hibidil Sterile Solution (CH), Hibiscrub (CH), Hibital alcoholic solution / tincture (CH), Hibitane concentrate (CH), Instillagel (D), Lifo-Scrub (CH), Lindosan wound and healing ointment (A), Luuf throat pastilles / throat spray (A), Merfen (CH), meridol med CHX 0.2% (D, CH), Neo-Angin Spray (CH), Nystalocal (D, CH), Paroex (D, F, CH, I), Paroguard (EU, CH), Perio-Aid (A, D, CH, E, NL, IT, UK), PerioChip (D, A, NL, CH, IT, UK), PlacAway (GR), Skinsept F / -mucosa (D), Uro-Tainer (CH), Vitaderm (A), Vita-Hexin (CH), Vita-Merfen (CH), Vitawund (A)
Web links
- Nicole B. Arweiler: The use of chlorhexidine for prophylaxis and therapy. (PDF; 6 MB) The most effective antibacterial agent in dentistry. In: http://www.izz-on.de/ . Udo Lenke, Ute Maier, May 2009, pp. 26–31 , accessed on March 29, 2016 .
Individual evidence
- ↑ Entry on CHLORHEXIDINE in the CosIng database of the EU Commission, accessed on December 28, 2019.
- ↑ a b Entry on chlorhexidine in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ a b c d Entry on chlorhexidine in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ Data sheet Chlorhexidine from Sigma-Aldrich , accessed on March 17, 2011 ( PDF ).
- ↑ G. De Lissovoy, AM Rentz, EM Dukes, CA Eaton, MK Jeffcoat, WJ Killoy, RD Finkelman: The cost-effectiveness of a new chlorhexidine delivery system in the treatment of adult periodontitis. In: J Am Dent Assoc. Volume 130, Number 6, June 1999, pp. 855-862, PMID 10377645 .
- ↑ NP Lang, M. Brecx: Chlorhexidine digluconate: an agent for chemical plaque control and preventions. In: J Periodontal Res. 21 (Suppl. 16), 1986, pp. 74-89. doi: 10.1111 / j.1600-0765.1986.tb01517.x
- ↑ A. Gaffar, J. Afflitto, N. Nabi: Chemical agents for the control of plaque and plaque microflora: an overview. In: Eur J Oral Sci. 14, 1997, pp. 502-507. PMID 9395116 .
- ↑ JG Elmore, RI Horwitz: Oral cancer and mouthwash use: evaluation of the epidemiologic evidence. In: Otolaryngology - Head and Neck Surgery . 113, 1995, pp. 253-261. PMID 7675486 .
- ↑ DM Winn, WJ Blot, JK McLaughlin, DF Austin, RS Greenberg, S. Preston-Martin, JB Schoenberg, Fraumeni JF : Mouthwash use and oral conditions in the risk of oral and pharyngeal cancer. In: Cancer Research . 51, 1991, pp. 3044-3047. PMID 2032242 .
- ↑ J. Llewelyn: Oral squamous cell carcinoma. Mouthwashes may increase risk. In: Br Med J . 308, 1994, p. 1508. PMID 8019293 .
- ↑ NB Arweiler, N. Boehnke, A. Sculean, E. Hellwig, TM Auschill: Differences in efficacy of two commercial 0.2% chlorhexidine mouthrinse solutions: a 4-day plaque re-growth study. In: J Clin Periodontol . 33, 2006, pp. 334-339. PMID 16634954 .
- ^ F. Bernardi, MR Pincelli, S. Carloni, MR Gatto, L. Montebugloni: Chlorhexidine with an anti discoloration system. A comparative study. In: Int J Dent Hyg. 2, 2004, pp. 122-126. PMID 16451475 .
- ↑ TM Kapellen, CM Gebauer, O. Brosteanu, B. Labitzke, C. Vogtmann, W. Kiess: Higher Rate of Cord-Related Adverse Events in Neonates with Dry Umbilical Cord Care Compared to Chlorhexidine Powder. In: Neonatology. 96, 2009, pp. 13-18, doi: 10.1159 / 000200165 .
- ↑ TM Kapellen, C. Gebauer, O. Brosteanu, B. Labitzke, W. Kiess, C. Vogtmann: Navel care: comparison of chlorhexidine powder with dry care. In: midwife. 22 (3), 2009, pp. 174-177, doi: 10.1055 / s-0029-1239958 .
- ^ RO Darouiche, MJ Wall Jr, KM Itani, MF Otterson, AL Webb: Chlorhexidine-Alcohol versus Povidone-Iodine for Surgical-Site Antisepsis. In: N Engl J Med. 362 (1), Jan 7, 2010, pp. 18-26. PMID 20054046 .
- ↑ LG Bode, JA Kluytmans, HF Wertheim, D. Bogaers, CM Vandenbroucke-Grauls: Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. In: N Engl J Med. 362 (1), Jan 7, 2010, pp. 9-17. PMID 20054045 .
- ↑ BfArM: Chlorhexidine-containing solutions for skin disinfection: skin burns in newborns. www.bfarm.de, medicines, pharmacovigilance, risk information, request for text adjustment (October 23, 2014) ( memento of November 10, 2014 in the Internet Archive ).
- ↑ EMA; PRAC recommendations on signals: Chlorhexidine cutaneous solutions - Chemical injury including burns when used in skin disinfection in premature infants. www.ema.europa.eu (September 25, 2014) .
- ^ P. Cortellini, A. Labriola, R. Zambelli, GP Prato, M. Nieri, MS Tonetti: Chlorhexidine with an anti discoloration system after peiodontal flap surgery: a cross-over, randomized, triple-blind clinical trial. In: J Clin Periodontol. 35, 2008, pp. 614-620. PMID 18422695 .
- ↑ Faber et al.: Allergy to chlorhexidine: beware of the central venous catheter. In: Acta Anaesthesiol Belg. 63 (4), 2012, pp. 191-194.
- ↑ LG Hjeljord, G. Rølla, P. Bonesvoll: Chlorhexidine-protein interactions. In: J Periodont Res. 8 (Suppl 12), 1973, pp. 11-16. PMID 4269593 .
- ^ HF Gilbert: Molecular and Cellular Aspects of Thiol-Disulfide Exchange. In: Advances in Enzymology and Related Areas of Molecular Biology. 63, 1990, pp. 69-172. doi: 10.1002 / 9780470123096.ch2
- ^ PC Jocelyn: Biochemistry of the SH Group. Academic Press, London / New York 1972, ISBN 0-12-385350-8 , p. 82.
- ↑ SK Grandhee, VM Monnier: Mechanism of formation of the Maillard protein cross-link pentosidine. In: J Biol Chem. 266 (18), 1991, pp. 11649-11653. PMID 4269593 .
- ^ M. Addy, WR Roberts: Comparison of the bisbiguanide antiseptics alexidine and chlorhexidine. II. Clinical and in vivo staining properties. In: J Clin Periodontol. 8, 1981, pp. 220-230. PMID 6947988
- ^ M. Addy, WG Wade, S. Jenkins, S. Goodfield: Comparison of two commercially available chlorhexidine mouthrinses: I. Staining and antimicrobial effects in vitro. In: Clin Prev Dent. 11, 1989, pp. 10-14. PMID 2638949 .
- ^ S. Jenkins, M. Addy, R. Newcombe: Comparison of two commercially available chlorhexidine mouthrinses: II. Effects on plaque reformation, gingivitis, and tooth staining. In: Clin Prev Dent. 11, 1989, pp. 12-16. PMID 2638954 .
- ↑ P. Cortellini, A. Labriola, R. Zambelli, GP Prato, M. Nieri, MS Tonetti: Chlorhexidine with an anti discoloration system after periodontal flap surgery: a cross-over, randomized, triple-blind clinical trial. In: J Clin Periodontol. 35, 2008, pp. 614-620. PMID 18422695 .
- ↑ NB Arweiler, N. Boehnke, A. Sculean, E. Hellwig, TM Auschill: Differences in efficacy of two commercial 0.2% chlorhexidine mouthrinse solutions: a 4-day plaque re-growth study. In: J Clin Periodontol. 33, 2006, pp. 334-339. PMID 16634954 .
- ↑ NB Arweiler, N. Boehnke, A. Sculean, E. Hellwig, TM Auschill: Differences in efficacy of two commercial 0.2% chlorhexidine mouthrinse solutions: a 4-day plaque re-growth study. In: J Clin Periodontol. 33, 2006, pp. 334-339. PMID 16634954 .
- ↑ P. Cortellini, A. Labriola, R. Zambelli, GP Prato, M. Nieri, MS Tonetti: Chlorhexidine with an anti discoloration system after periodontal flap surgery: a cross-over, randomized, triple-blind clinical trial. In: J Clin Periodontol. 35, 2008, pp. 614-620. PMID 18422695 .
- ↑ NB Arweiler, N. Boehnke, A. Sculean, E. Hellwig, TM Auschill: Differences in efficacy of two commercial 0.2% chlorhexidine mouthrinse solutions: a 4-day plaque re-growth study. In: J Clin Periodontol. 33, 2006, pp. 334-339. PMID 16634954 .
- ^ PS Roland, JA Rutka: Ototoxicity. BC Decker, Hamilton, Ontario 2004, ISBN 1-55009-263-4 .
- ↑ Red List, as of August 2009.
- ↑ AM comp. d. Switzerland, as of August 2009.
- ↑ AGES-PharmMed, as of August 2009.