Benoxaprofen
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
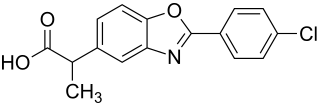
|
||||||||||||||||||||||
| Structural formula without stereochemistry: 1: 1 mixture ( racemate ) of ( R ) form and ( S ) form |
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Benoxaprofen | |||||||||||||||||||||
| other names |
( RS ) -2- [2- (4-chlorophenyl) -5-benzoxazoyl] propionic acid ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 16 H 12 ClNO 3 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 301.72 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
189-190 ° C |
|||||||||||||||||||||
| pK s value |
3.5 |
|||||||||||||||||||||
| solubility |
Water: 52 mg l −1 at 25 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Benoxaprofen is a chlorine- containing, heterocyclic compound, which is derived from oxazole and propionic acid. As a medicinal substance, benoxaprofen, like the similar ibuprofen, belongs to the group of non-steroidal anti-inflammatory drugs or anti-inflammatory drugs . The drug was developed by Eli Lilly in the 1980s and used as an analgesic , antipyretic and anti-inflammatory drug . After severe side effects such as strong allergic reactions ( photosensitivity ) to the drug and liver damage with hundreds of deaths occurred in Great Britain , the drug's approval was withdrawn again. The former preparation Oraflex ® is no longer available.
Appearance and properties
The waxy white substance belongs to the group of propionic acid derivatives. It is produced from 4-aminophenyl-α-methylacetonitrile after nitration and hydrogenation by reaction with pyridine and subsequent reaction with hydrogen chloride . Benoxaprofen was used in medicines as the sodium salt.
Individual evidence
- ↑ a b c Bruchhausen, Ebel, Frahm, Hackenthal: Hagers Handbook of Pharmaceutical Practice. Folgewerk, Vol. 7, pp. 403-404, 1999, Springer-Verlag, ISBN 3-540-52632-3 .
- ↑ a b Entry on benoxaprofen in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Yakuri to Chiryo. in: Pharmacology and Therapeutics. Vol. 9, p. 4445, 1981 .
- ^ Journal of Medicinal Chemistry. Vol. 18, p. 53, 1975 .
- ↑ Nephron. Vol. 35, p. 279, 1983 .
- ^ David Coburn, Carl D'Arcy, George Murray Torrance: Health and Canadian Society: Sociological Perspectives. 3rd edition, University of Toronto Press, 1998, ISBN 978-0-8020-8052-3 , p. 487 ( limited preview in Google book search).