Cesium chloride
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| __ Cs + __ Cl - | |||||||||||||||||||
| Crystal system |
cubic |
||||||||||||||||||
| Space group |
Pm 3 m (No. 221) |
||||||||||||||||||
| Lattice parameters |
412.6 pm (at T = 298 K) |
||||||||||||||||||
| Coordination numbers |
Cs [8], Cl [8] |
||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Cesium chloride | ||||||||||||||||||
| Ratio formula | CsCl | ||||||||||||||||||
| Brief description |
white, odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 168.36 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
3.97 g cm −3 |
||||||||||||||||||
| Melting point |
646 ° C |
||||||||||||||||||
| boiling point |
1382 ° C |
||||||||||||||||||
| solubility |
very good in water (1860 g l −1 at 20 ° C) |
||||||||||||||||||
| Refractive index |
1.640 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
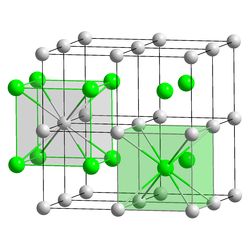
Cesium chloride , a salt with the formula CsCl, forms colorless, cubic crystals ( cesium chloride structure ) and can be dissolved in water , acids and alkalis . The cesium cations and chloride anions can move freely in solution, and the mobility of the cesium ion in water is relatively high, since the cesium ion has only a relatively weakly bound hydrate shell . Cesium chloride is hygroscopic .
structure
Cesium chloride has a characteristic, cubic crystal structure in which each cesium ion is surrounded by eight chloride ions and each chloride ion is surrounded by eight cesium ions: The cubic unit cell can, for example, be selected so that the chloride ions are located at the eight corners of the cube. Then the cesium ion is exactly in the middle of the cube. Alternatively, you can choose a unit cell in which the cesium ions are in the corner of the cube and the chloride ion is in the middle of the cell. The cesium chloride structure with the relatively high coordination number eight is only formed in alkali metal halides if the cation is relatively large, so that it can also be surrounded by eight anions . Otherwise the sodium chloride structure will form . Even brass with a zinc content of around 50% has the cesium chloride structure at temperatures below 468 ° C.
properties
The standard enthalpy of formation of cesium chloride is Δ H f 0 = −443 kJ / mol.
presentation
Cesium chloride can be synthesized using cesium hydroxide or cesium carbonate , together with hydrochloric acid :
or
use
Use in research
Cesium chloride is often used in laboratory processes for purification of nucleic acids or viruses using an ultra-centrifuge used. After several hours of ultracentrifugation, a density gradient forms automatically. The particles then collect in characteristic bands in the centrifuge tube according to their density and can be removed separately.
It is also used in atomic absorption spectrometry as an ionization buffer for easily ionizable metals.
Misuse
In pseudomedicine , cesium chloride is used to treat cancer. Supporters of so-called cesium chloride therapies speculatively assume that the pH value in the cytoplasm of tumor cells is lower than in healthy cells. Cesium chloride is supposed to raise the pH value in the tumor cells to the normal value, which is why this therapy is also called high pH therapy in English . Furthermore, cesium should lead to tumor cells being able to absorb less glucose and thus “starve”. A scientifically recognized proof of the pharmacological effectiveness could not be provided.
Individual evidence
- ↑ V. Ganesan, KS Girirajan, Lattice parameters and thermal expansion of CsCl and CsBr by x-ray powder diffraction. I. Thermal expansion of CsCl from room temperature to 90 K , Pramana - J. Phys. 27: 472 (1986)
- ↑ a b c d e f data sheet cesium chloride (PDF) from Merck , accessed on January 19, 2011.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Index of Refraction of Inorganic Crystals, pp. 10-246.
- ↑ a b Entry on cesium chloride in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 1170.
- ↑ Chemgapedia, Cesium Chloride, page 3: Synthesis , accessed on January 17, 2015 at 9:50 pm
- ↑ Cesium Chloride ( Memento November 12, 2006 in the Internet Archive ) In: American Cancer Society . June 1, 2005, accessed February 9, 2019.



