Cladribine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
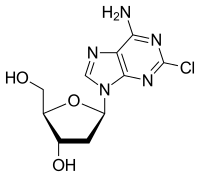
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Cladribine | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 10 H 12 ClN 5 O 3 | |||||||||||||||||||||
| Brief description |
white to almost white, crystalline , polymorphic powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 285.69 g mol −1 | |||||||||||||||||||||
| solubility |
soluble in dimethyl sulfoxide ; sparingly soluble in water, methanol ; practically insoluble in acetonitrile |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Cladribine is a drug with antineoplastic and immunomodulating effects that is approved , among other things, for the oral treatment of multiple sclerosis (MS) . Cladribine was originally developed and approved for the treatment of hairy cell leukemia , a form of blood cancer. In this indication it is given parenterally .
Clinical information
application areas
multiple sclerosis
Cladribine tablets ( Mavenclad / Merck KGaA ) are used to treat adult patients with highly active relapsing multiple sclerosis (MS) as defined by clinical or imaging findings.
Haematological diseases
Cladribine solution for infusion and injection ( Leustatin / Janssen-Cilag ; Litak / Lipomed) for intravenous or subcutaneous administration are approved in the EU for the treatment of hairy cell leukemia .
Type and duration of application
Cladribine, the first approved in Europe oral pulse therapy . Is approved for relapsing MS While any short-term ingestion phase ( "pulse") once a day - depending on body weight - one or two tablets during the first four or five days taken one month and the beginning of second month repeated. The treatment phase in the 2nd year is important for the success of the treatment. It takes place in the same way as in the first year. In total, this results in a maximum of 20 treatment days in the first two years.
After the two years of treatment have been completed, no further treatment with cladribine is required in years 3 + 4, since in a clinical study 75.6% of the patients were consistently relapse-free in years 3 + 4. From year 5, further treatment with cladribine tablets can be added.
Contraindications
Infection with the human immunodeficiency virus (HIV). Active chronic infection ( tuberculosis or hepatitis ).
Drug interactions
Due to the risk of additive effects on the immune system, initiating treatment with cladribine is contraindicated in immunocompromised patients , including patients currently receiving immunosuppressive or myelosuppressive therapy with agents such as methotrexate , cyclophosphamide , ciclosporine, or azathioprine , and in patients on chronic treatment with corticosteroids .
Use during pregnancy and breastfeeding
Men and women must use effective contraception during treatment with cladribine and for at least six months after the last dose. This is important because cladribine could potentially cause serious harm to the fetus. It is not known whether cladribine is excreted in breast milk; therefore, breastfeeding should not be carried out for up to a week after taking it.
unwanted effects
The most common side effects in patients with multiple sclerosis treated with cladribine tablets were a decrease in the number of lymphocytes ( lymphopenia ) and certain white blood cells (neutrophils; neutropenia ) and oral herpes / shingles ( herpes zoster ). Skin rashes and hair loss ( alopecia ) were also common. Lymphocyte counts normalized over time in most patients; severe lymphopenia (grade 4) was observed in less than 1% of patients.
At the world's largest annual international congress dedicated to basic and clinical research in multiple sclerosis, ECTRIMS, the results of post-approval data on safety were presented in a poster presentation in 2019 (integrated analysis from the clinical study program with oral cladribine; Market surveillance after approval ( post marketing reporting ). As a result, the spectrum of side effects is consistent with that at the time of approval, no new signals (i.e. possible causal relationship between drug use and an event ) were detected.
After parenteral administration for the treatment of hairy cell leukemia, cladribine can cause toxic effects such as bone marrow suppression and lymphocytopenia, as well as immunosuppression and opportunistic infections, because of its antineoplastic and immunosuppressive effects . Cases of progressive multifocal leukoencephalopathy (PML) have been reported. The usual side effects associated with the use of cytostatic drugs, such as fever, nausea, loss of appetite and dizziness, also occur.
Studies on the treatment of MS
- Cladribine has been studied in numerous multiple sclerosis clinical studies. The substance showed significant effects on the relevant disease parameters MRI , relapse activity and disease progression.
- In patients with relapsing-remitting MS, cladribine in the CLARITY study showed significant effects on clinical and MRI parameters compared to placebo : a reduction in the annual relapse rate (relapse-free in 77.8% of patients in years 1 + 2 and in 75.6 % of patients in year 3 + 4; reduction in relative risk by 58% after two years), an increase in the relapse-free period, a reduction in the risk of disability progression confirmed after three months (reduction in the relative risk by 33%) and a reduction in different lesions measured on MRI. After 96 weeks, 47% of the patients showed no measurable disease activity (NEDA).
- Post-hoc analyzes of the two-year Phase III CLARITY study showed that cladribine tablets reduced the annual relapse rate by 67% and the risk of 6-month confirmed disability progression - by 82% compared to placebo in patients with high disease activity *.
- The ORACLE-MS study examined the efficacy and tolerability of cladribine compared to placebo in patients with a first demyelinating event (FCDE). Cladribine significantly reduced the relative risk of developing clinically manifest MS by 67%.
- In the randomized phase IIb study ONWARD, the efficacy and tolerability of cladribine as an add-on therapy to interferon beta in patients with at least one relapse in the last 48 weeks were compared with placebo plus interferon beta over two years. Patients who were also treated with cladribine tablets had a 63% reduced risk of a relapse.
Mechanism of action
Cladribine is a chlorinated analogue of the DNA building block deoxyadenosine . It is a so-called prodrug that is absorbed by cells with the help of specific transporter proteins ( nucleoside transporters ). The inactive prodrug is preferably phosphorylated in lymphocytes and converted into its active form, 2-chlorodeoxy-adenosine-5'-triphosphate (2-CdATP). 2-CdATP works by being incorporated into the DNA or RNA as the wrong building block . The double helix structure is disrupted and DNA repair and DNA synthesis are inhibited. This leads to a targeted reduction in the number of lymphocytes. Cladribine acts in both dividing ( proliferating ) and resting cells.
In the treatment of multiple sclerosis (MS) Cladribine acts as a so-called disease-modifying drugs ( disease-modifying drug , DMD). Therapy with cladribine selectively and temporarily reduces the number of T and B lymphocytes , which are significantly involved in the disease process of MS. The lymphocytes are then repopulated, with the effect of cladribine remaining sustained even after the lymphocyte count has recovered. Since cladribine primarily reduces the number of T and B lymphocytes and has only minor or transient effects on monocytes , neutrophils and natural killer cells, the innate immune defense is preserved.
In addition, there are indications from studies that cladribine shifts the cytokine profile from pro-inflammatory towards anti-inflammatory by increasing the concentration of some anti-inflammatory cytokines (interleukin-4, interleukin-5 and interleukin-10) and the levels of pro-inflammatory TNF-α and interleukin-6 normalizes and lowers the concentration of TGF-ß1 and basic fibroblast growth factor.
Cladribine can cross the blood-brain barrier . In the cerebrospinal fluid of patients who do not suffer from a disease of the central nervous system, concentrations of about 25% of the plasma level are reached.
Development and marketing
Cladribine was developed by Ernest Beutler's research group at the Scripps Research Institute in the 1970s and was first studied in nine patients with treatment-resistant blood cancer in the early 1980s . In 1988 the same working group reported on the first treatment of patients with hairy cell leukemia. Corresponding cladribine preparations for intravenous treatment ( leustatin ) were approved in 1993 in the USA and Sweden, and later also in other countries, including Germany (1996). In some countries the indications also included certain other malignant lymphatic diseases.
In 2004, a subcutaneous injection solution ( litak ) for the treatment of hairy cell leukemia was approved throughout the EU . Development began in Switzerland in the 1990s with the aim of making therapy more comfortable for the patient. The subcutaneous administration, which is required for 5 consecutive days, can be carried out by the patient himself. The exposure is similar to that of intravenous administration. Subcutaneous injection of Litak was approved by the European Commission as a drug approved for orphan.
Beutler and colleagues also studied cladribine in patients with multiple sclerosis in the 1990s. This treatment approach was later taken up by Merck Serono . European approval for cladribine tablets for the treatment of adult patients with highly active relapsing MS, as defined by clinical and imaging findings, was granted in August 2017. An initial application for approval from Merck in 2011 failed because the Committee for Medicinal Products for Human Use (CHMP) was of the opinion stated "that based on the data available at the time, the benefits of cladribine tablets do not outweigh the risks of treating multiple sclerosis." In the United States, cladribine was approved for the treatment of MS on March 29, 2019.
Cladribine is also being studied for the treatment of mantle cell lymphoma and other B-cell lymphomas. For the indication mastocytosis it has the status of an orphan medicinal product in the EU.
Early benefit assessment
In Germany, since 2011, newly approved drugs with new active ingredients must be subjected to an " early benefit assessment " by the Federal Joint Committee (G-BA) in accordance with Section 35a SGB V if the pharmaceutical manufacturer wants to achieve a higher sales price than just the fixed amount . Only if there is an additional benefit can the pharmaceutical manufacturer negotiate a price with the umbrella association of statutory health insurance companies. The dossier evaluations, on the basis of which the G-BA makes its decisions, are created by the Institute for Quality and Efficiency in Health Care (IQWiG) .
In the early benefit assessment in 2018, a distinction was made between adults with highly active relapsing multiple sclerosis who had not yet received disease-modifying therapy and those with highly active disease despite treatment with disease-modifying therapy. According to the G-BA decision, an additional benefit has not been proven for either of these two groups.
chemistry
Cladribine has three stereogenic centers. Two stereoisomers are possible, the 9-α and 9-β form, with cladribine representing the 9-β stereoisomer. 2-Chloro-9- (2-deoxy-α-D-erythro-pentofuranosyl) -9 H -purine-6-amine occurs as an impurity in small amounts (maximum 0.2 percent).
Trade names
- Multiple Sclerosis: Mavenclad (10 mg tablets)
- Haematological diseases: Leustatin (10 mg / 10 ml concentrate for solution for infusion), Litak (2 mg / ml solution for injection)
Web links
- Entry for Cladribine in the Human Metabolome Database (HMDB) , accessed October 17, 2013.
- Entries in the NIH study registry
Individual evidence
- ↑ a b c European Pharmacopoeia Commission (Ed.): European Pharmacopoeia 8th Edition . 8.0 (basic work), 2014.
- ↑ a b Data sheet 2-Chloro-2′-deoxyadenosine from Sigma-Aldrich , accessed on March 23, 2011 ( PDF ).
- ↑ Entry on cladribine in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ a b c d e f g h i Mavenclad: Summary of Product Characteristics (Product Information). (PDF) Retrieved April 19, 2019 .
- ↑ Hairy cell leukemia (HZL): Onkopedia guideline. German Society for Hematology and Medical Oncology, April 2016, accessed on May 16, 2018 .
- ↑ a b c d e Giovannoni G, Comi G, Cook S et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010; 362: 416-426, doi: 10.1056 / NEJMoa0902533 (free full text). PMID 20089960 .
- ↑ on the study program see: Assessment Report Mavenclad , CHMP June 22, 2017, Table 2 , ema.europa.eu (PDF).
- ↑ Updated safety of cladribine tablets in the treatment of patients with multiple sclerosis: Integrated safety analysis and post-approval data , ECTRIMS Kongress, Stockholm September 2019, accessed April 10, 2020
- ↑ a b c Summary of the characteristics of the drug Litak . (PDF) EMA (specialist information); accessed on March 20, 2019.
- ↑ Cook S, Vermersch P, Comi G et al. Safety and tolerability of cladribine tablets in multiple sclerosis: the CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) study. Mult Scler. 2011 May; 17 (5): 578-593, PMID 21228029 ; doi: 10.1177 / 1352458510391344 .
- ↑ a b Gavin Giovannoni, Stuart Cook, Kottil Rammohan, Peter Rieckmann, Per Soelberg Sørensen: Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis . In: The Lancet Neurology . tape 10 , no. 4 , p. 329-337 , doi : 10.1016 / s1474-4422 (11) 70023-0 .
- ↑ Giancarlo Comi, Stuart D. Cook, Gavin Giovannoni, Kottil Rammohan, Peter Rieckmann: MRI outcomes with cladribine tablets for multiple sclerosis in the CLARITY study . In: Journal of Neurology . tape 260 , no. 4 , April 1, 2013, p. 1136–1146 , doi : 10.1007 / s00415-012-6775-0 .
- ↑ Thomas P Leist, Giancarlo Comi, Bruce AC Cree, Patricia K Coyle, Mark S Freedman: Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomized trial . In: The Lancet Neurology . tape 13 , no. 3 , p. 257-267 , doi : 10.1016 / s1474-4422 (14) 70005-5 .
- ↑ Montalban X, Cohen B, Leist T, et al .: Efficacy of Cladribine Tablets as Add-On to IFN-beta Therapy in Patients with Active Relapsing MS: Final Results from the Phase II ONWARD Study (P3.029) . In: Neurology . tape 86 , 16 Supplement, 2016, pp. P3.029 ( neurology.org ).
- ^ Thomas P. Leist, Robert Weissert: Cladribine. In: Clinical Neuropharmacology. 34, 2011, p. 28, doi: 10.1097 / WNF.0b013e318204cd90 .
- ↑ G. Giovannoni et al .: AAN 2016 . P3.028.
- ↑ G. Giovannoni et al .: EAN 2017 . P0542.
- ^ Thomas P. Leist, Robert Weissert: Cladribine . In: Clinical Neuropharmacology . tape 34 , no. 1 , p. 28-35 , doi : 10.1097 / wnf.0b013e318204cd90 .
- ↑ Melanie Korsen, Sara Bragado Alonso, Lizzy Peix, Barbara M. Bröker, Alexander Dressel: Cladribine Exposure Results in a Sustained Modulation of the Cytokine Response in Human Peripheral Blood Mononuclear Cells . In: PLOS ONE . tape 10 , no. 6 , June 18, 2015, p. e0129182 , doi : 10.1371 / journal.pone.0129182 .
- ↑ Achille Aouba, Benjamin Terrier, Viorel Vasiliu, Sophie Candon, Nicole Brousse: Dramatic clinical efficacy of cladribine in Rosai-Dorfman disease and evolution of the cytokine profile: towards a new therapeutic approach . In: Haematologica . tape 91 , 12 Suppl, December 2006, pp. ECR52 , PMID 17194658 .
- ↑ J. Gora-Tybor, JZ Blonski, T. Robak: Cladribine decreases the level of angiogenic factors in patients with chronic lymphocytic leukemia . In: neoplasm . tape 49 , no. 3 , 2002, p. 145-148 , PMID 12097998 .
- ↑ January lily Mark: The Clinical Pharmacokinetics of Cladribine . In: Clinical Pharmacokinetics . tape 32 , no. 2 , February 1, 1997, p. 120-131 , doi : 10.2165 / 00003088-199732020-00003 .
- ^ DA Carson, DB Wasson, E. Beutler: Antileukemic and immunosuppressive activity of 2-chloro-2'-deoxyadenosine . In: Proceedings of the National Academy of Sciences . tape 81 , no. 7 , 1984, pp. 2232-2236 , doi : 10.1073 / pnas.81.7.2232 , PMID 6585795 .
- ↑ LD Piro, CJ Carrera, E. Beutler, DA Carson: 2-Chlorodeoxyadenosine: an effective new agent for the treatment of chronic lymphocytic leukemia . In: Blood . tape 72 , no. 3 , 1988, pp. 1069-1073 , PMID 2901280 .
- ↑ a b European Public Assessment Report Litak - Scientific Discussion. (PDF; 417 kB) European Medicines Agency, October 21, 2005; Retrieved October 2, 2010.
- ↑ Human medicinal products with new active ingredients (1996) . In: Federal Health Gazette . tape 40 , no. 4 , 1997, p. 151-153 , doi : 10.1007 / BF03044171 .
- ↑ JC Sipe, JS Romine, JA Koziol, et al .: Development of cladribine treatment in multiple sclerosis . In: Multiple Sclerosis Journal . tape 1 , no. 6 , 1996, pp. 343-347 , doi : 10.1177 / 135245859600100612 , PMID 9345414 .
- ↑ European Commission grants approval for Mavenclad (cladribine tablets). Press release. Merck , accessed March 18, 2019 .
- ↑ Julia Borsch: Cladribine receives approval for MS in the second attempt. In: deutsche-apotheker-zeitung.de. August 28, 2017, accessed March 30, 2019 .
- ↑ FDA: FDA approves new oral treatment for multiple sclerosis. Retrieved April 10, 2020 .
- ^ Inwards DJ, Fishkin PA, Hillman DW et al. Long-term results of the treatment of patients with mantle cell lymphoma with cladribine (2-CDA) alone (95-80-53) or 2-CDA and rituximab (N0189) in the North Central Cancer Treatment Group. Cancer. 2008; 113: 108-116. PMID 18470909 ; doi: 10.1002 / cncr.23537 .
- ^ Sigal DS, Miller HJ, Schram ED, Saven A. Beyond hairy cell: the activity of cladribine in other hematologic malignancies. Blood. 2010; Jul 15. PMID 20634380 .
- ↑ Entry EU / 3/13/1182 in the Community Register for Orphan Medicines from August 2013.
- ↑ A17-62 Cladribine (multiple sclerosis) - Benefit assessment according to Section 35a SGBV; Accessed March 27, 2020.
- ↑ Benefit assessment procedure for the active ingredient cladribine (highly active relapsing multiple sclerosis); Accessed March 27, 2020.
- ↑ Assessment Report Mavenclad . (PDF) Committee for Medicinal Products for Human Use (CHMO), June 22, 2017
- ↑ Janssen-Cilag: Technical information Leustatin 10 mg / 10 ml concentrate for the preparation of an infusion solution (PDF) as of December 2017.