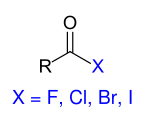Derivative (chemistry)
In organic chemistry, a substance can be designated as the derivative (from Latin derivare , to derive ) of a parent compound (basic substance, parent substance) if it has a structural unit that is similar to the functional group of the parent compound and is a structural element of this functional group contains the same oxidation state . Often derivatives can be prepared from the respective parent compound in a single reaction step. Activation of the functional group of the parent compound can precede this one reaction step. One then speaks of reactive derivativesthe parent compound in question, which can also be isolated if necessary and from which further derivatives can then be easily prepared. Well known as reactive derivatives of carboxylic acids are the carboxylic acid halides . Chemical reactions for the production of derivatives are known as derivatization . In the case of complex compounds with several identical or different functional groups, a targeted derivatization reaction on a specific functional group is often not possible without first blocking the other functional groups with protective groups .
According to the definition, the parent compound and its derivatives are structurally closely related. Instead of the functional group of the parent compound, a new functional group is found in the derivative, but this has a structural sub-element of the functional group of the parent compound in the same oxidation state. Because of the new functional group, derivatives usually have significantly different chemical and physical properties than the parent compounds, but can be converted back into the parent compound with suitable reactions.
Definition of the term derivative
Contrary to the strict definition of the term derivative formulated in the introduction, it is often used in common parlance as a structural derivative of a basic substance. Substances that have a similar structure or have the structure of the basic substance as a partial structure are then referred to as derivatives of the basic substance. The term derivative is used in these cases with the intention of referring to these similarities or to the partial structural identity of the descendant with the structure of a possibly well-known parent compound.
Examples of derivatives
Carboxylic acid derivatives
The group of carboxylic acid derivatives is the best way to find out which compounds meet the strict requirements for a derivative formulated in the introduction. Carboxylic acid derivatives are carboxylic acid halides , carboxylic acid anhydrides , carboxylic acid esters , carboxylic acid amides , carboxylic acid hydrazides and carboxylic acid azides . The carboxylic acid derivatives differ formally from the underlying parent compound carboxylic acid in that only the OH group of the carboxy group has been replaced (substituted) by another group beginning with a heteroatom (usually: Hal, O, S, N). As an essential structural element of the functional carboxyl group, the carbonyl group of the carboxyl group remains in the same oxidation state and can also determine the reaction behavior of the carboxylic acid derivatives. In all cases, the derivatization reactions for the preparation of the derivatives can be reversed again by hydrolysis reactions . The relative reactivity of the carboxylic acid derivatives decreases in the sequence shown below. This can be justified by the fact that the electronegativity of the substituents marked in blue also decreases in this order, which is associated with a decreasing exit capacity.
More examples of derivatives
Typical examples of derivatives as defined in the introduction:
- From a formal point of view, chloroform is a derivative of formic acid or the unstable formyl chloride, depending on the oxidation state of one of the carbon atoms .
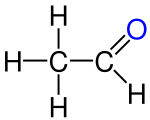
- From a formal point of view, ethanol and ethanal are derivatives of monochloroethane or 1,1-dichloroethane only according to their oxidation state .
- Diethyl ether is a derivative of ethanol in which the OH group has been replaced by an O-alkyl group (OR group) without changing the oxidation state. When the derivative is formed (derivatization reaction) with H 2 SO 4 , an alkyl sulfate is initially formed as a reactive intermediate.
- Imines and oximes are derivatives of aldehydes and ketones, formed by their reactions with amines or hydroxylamine.
- Hydrazones are derivatives of aldehydes and ketones, formed by their reactions with hydrazine or its derivatives.
- Disaccharides are derivatives of two monosaccharides . Polysaccharides such as B. Starch are derivatives of monosaccharides, e.g. B. Glucose Derivatives. When glycosidic bonds are formed , an OH group is replaced by an O-alkyl group without changing the oxidation state. The OH groups in polysaccharides such as starch can be mixed with reactive carboxylic acid derivatives, e.g. B. with acetyl chloride , derivatized. The resulting products are called derivatized or modified starch .
- As carboxylic acid esters, fats are derivatives of glycerine and are accordingly referred to as triglycerides . In addition, fats are also derivatives of the carboxylic acids that are typical for the respective fat.
- Fats that contain the structural element of phosphorylated glycerine are known as phosphoglycerine derivatives ( phosphoglycerides ).
- Lipids that contain the structural element sphingosine (a monounsaturated amino alcohol) are known as sphingosine derivatives or sphingomyelins .
Examples of structural descendants
Typical examples of structural descendants that are not derivatives in the sense of the above definition, but which are sometimes also referred to as derivatives in common parlance.
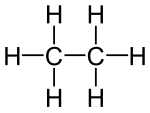
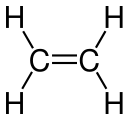
Structural derivatives of methane and ethane
- Chloroform is a structural derivative of methane because both compounds contain a single C atom and because three H atoms have been replaced by Cl atoms. However, the carbon atom in both compounds is in a different oxidation state.
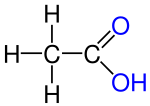
- Ethanol , ethanal, ethene, ethyne, acetic acid, chloroethane are structural derivatives of ethane , as all compounds contain two linked carbon atoms. In fact, the carbon atoms in the structural descendants are in higher, different oxidation states than in the ethane itself. All compounds cannot be produced from ethane by derivatization reactions, but only by more or less complex syntheses, which are always associated with an oxidation of the ethane .
According to the strict definition in the introduction, the compounds shown are not derivatives of ethane, although they all have two carbon atoms. The compounds are independent compounds with new functional groups, with the carbon atoms mostly in higher oxidation states compared to ethane. The preparation of these compounds from ethane is not a derivatization reaction but a synthesis .
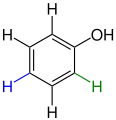
Structural derivatives of phenol
In common usage, the capsaicin contained in chilli and the good-smelling raspberry ketone are called derivatives of phenol , as these compounds contain the partial phenol structure. However, since both compounds contain additional functional groups and the original OH group of phenol is unchanged, these are not derivatives as defined above, but only structurally related compounds.
More examples of misleading use of the term derivative
- Phenylhydrazine is sometimes referred to as a chemical compound from the group of hydrazine derivatives. In fact, phenylhydrazine is an independent compound, just as aniline as an independent amine is not a compound from the “group of ammonia derivatives”.
- It is sometimes claimed that α-aminocarboxylic acids and α-chlorocarboxylic acids are derivatives of carboxylic acids because both classes of compounds contain the functional carboxy group typical of carboxylic acids as an important structural element . In fact, however, it is a matter of different classes of compounds, each with two functional groups, one of which is identical. Therefore, aminocarboxylic acids and chlorocarboxylic acids are better described as structural derivatives of carboxylic acids. On the other hand, aminocarboxylic acids can be called derivatives of chlorocarboxylic acids, because it is possible at least in a formal derivatization reaction to replace the functional group C-Cl in the chlorocarboxylic acid by reacting with ammonia NH 3 with the new functional group C-NH 2 in the same oxidation state.
The importance of derivatization
For certain purposes, derivatives can be produced very specifically from the parent compounds (basic substance) with the help of derivatization reactions. Then derivatives should meet certain additional requirements that are not met by the basic substance. The following areas of application can be defined:
- Pharmacy: In the case of active ingredients , derivatives should be better tolerated or effective than the basic substance.
- Separation: Derivatives should have better or worse solubility in order to be able to separate them from other substances by filtration or centrifugation .
- Analysis, separation, identification: derivatives should have higher volatilities than the basic substances so that they can be used e.g. B. analytically separate and identify by means of gas chromatography . Derivatives should be colored so that they can be recognized in thin layer chromatography . Derivatives should be solid and have defined melting points so that the associated parent compounds can be clearly identified.
- Synthesis: Derivatives should have a higher reactivity than the parent compounds, so that new bonds can be made more easily with these so-called reactive derivatives than from the parent compounds and new substances can be synthesized.
pharmacy
In pharmacy , derivatization can be of particular importance in order to make existing drugs more effective or better tolerated. So you can z. B. the acetylsalicylic acid both as a derivative of acetic acid (an ester) and as a derivative of the phenolic OH group of salicylic acid . By acetylation , d. H. By converting it into a derivative of acetic acid, the inadequate analgesic effect of salicylic acid could be improved.
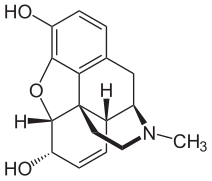
Another example of the change in the effectiveness of a medicinal product through derivatization is the conversion of morphine into heroin , which, as 3,6-diacetylmorphine, is an acetic acid derivative of morphine and is obtained from morphine by ( acetylation ).
Chromatography
Derivatization plays an important role in the entire chromatographic analysis.
In gas chromatography and gas chromatography with mass spectrometry coupling , derivatives are mostly used in order to convert analytes which cannot be evaporated or which are difficult to evaporate into more volatile derivatives which are accessible to chromatography in the gas phase .
In HPLC analysis , chromophoric and / or fluorescent derivatives are often used to enable sensitive and specific detection in the visible or ultraviolet spectral range . In thin layer chromatography , substances can be made visible with detection reagents with the formation of colored derivatives. This is known as post-chronmatographic derivatization . The derivatization reagents are sprayed onto the TLC plates or the TLC plates are vaporized with the derivatization reagent or immersed in it.
Common derivatization reagents in chromatography are
- Ninhydrin for the detection of amino acids
Characterization of connections
Derivatization has historical importance in the analysis and characterization of organic compounds:
- The hydrazones which crystallize particularly well are obtained by reacting carbonyl compounds with 2,4-dinitrophenylhydrazine .
- The method of identifying monosaccharides by derivatizing them with phenylhydrazine to form the osazones was developed by Emil Fischer in 1884.
Homologous series
The term homologue must be distinguished from the term derivative . Homologs are substances that differ only in the chain length of their basic building blocks; in organic chemistry, these are the hydrocarbon chains of alkanes , alkenes , alcohols or carboxylic acids. Members of this homologous series are often very similar in their chemical and physical properties. As a rule, homologs are not derivatives.
literature
- Karl Blau, Graham S. King (Ed.): Handbook of Derivatives for Chromatography. Heyden & Son Ltd., London 1977, ISBN 0-85501-206-4 .
- RW Frei, JF Lawrence (Ed.): Chemical Derivatization in Analytical Chemistry. Volume 1: Chromatography. Plenum Press, New York & London 1981, ISBN 0-306-40608-X .
- Entry on derivatives. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- Daniel R. Knapp: Handbook of Analytical Derivatization Reactions. J. Wiley & Sons, New York 1997, ISBN 0-471-03469-X .
- Kurt Peter C. Vollhardt : Organic chemistry (= Organic chemistry, structure and function ). 3rd edition Wiley-VCH, Weinheim 2000, ISBN 3-527-29819-3 (Chapters 15, 19 and 20).
Individual evidence
- ^ Brockhaus ABC chemistry. VEB FA Brockhaus Verlag Leipzig 1965, p. 276.
- ↑ a b Entry on derivatisation. In: Römpp Online . Georg Thieme Verlag, accessed on November 8, 2018.
- ↑ Oliver Reiser: Organic Chemistry: Study compact . Ed .: Paula Y. Bruice. 5th edition. Pearson Studium, Munich 2011, ISBN 978-3-86894-102-9 , pp. 658 .
- ↑ K. Peter C. Vollhardt, Neil E. Schore: Organic chemistry . Ed .: Holger Butenschön. 5th edition. Wiley-VCH, Weinheim 2011, ISBN 978-3-527-32754-6 , pp. 991-993 .
- ↑ DocCheck Flexikon: Derivatization , accessed on November 20, 2018.
- ↑ Hans Beyer , Wolfgang Walter : Textbook of organic chemistry . 18th edition. S. Hirzel Verlag, Stuttgart 1978, ISBN 3-7776-0342-2 .
- ^ Brockhaus ABC chemistry. VEB FA Brockhaus Verlag Leipzig 1965, p. 551.