Chlor-alkali electrolysis

With chlor-alkali electrolysis , the important basic chemicals chlorine , hydrogen and caustic soda are produced from sodium chloride and water . The term generally stands for production by means of electrolysis, regardless of which of the techniques is used. Other metal salts (chlorides of alkali metals , alkaline earth metals and many more) can also be converted into their hydroxides by means of electrolysis . For example, if you use potassium chloride instead of sodium chloride, you get potassium hydroxide . Chloralkali electrolysis is an endothermic reaction . The required energy of 454 kJ / mol is supplied in the form of electrical current . Since caustic soda, chlorine and other basic chemicals are required on a scale of millions of tons annually, electrolysis is extremely energy-intensive. Hydrogen as a product is just as important, but the process is mainly used for the production of chlorine and caustic soda. Modern processes prevent the reduction of water to hydrogen and thus save considerable amounts of energy. In all processes, an attempt is made to separate the chlorine gas from the hydroxide in order to avoid disproportionation of chlorine to chloride and hypochlorite , which would contaminate the sodium hydroxide solution. The recovered amounts of caustic soda are often evaporated to obtain solid sodium hydroxide .
history
At the end of the 19th century, the three processes for the industrial production of chlorine and sodium and potassium hydroxide were the diaphragm process, the bell process and the amalgam process.
The diaphragm process was developed by Breuer in 1885. Ignatz Stroof and the Lang brothers built the first large-scale plant for the production of caustic soda and chlorine using the diaphragm process at the Elektron chemical factory in Griesheim . The Griesheim-Elektron company was founded in 1898. Three large plants were built in Germany, namely in Griesheim, Bitterfeld and Greppin , as well as several plants in other European countries (Spain, France, Russia). In France, a modified diaphragm method was worked out by Outhenin, Chalandres. However, operations soon had to be stopped.
The amalgam process is also known as the Castner-Kellner process (sodium hydroxide) and was invented in the 1890s by Hamilton Castner in England and Karl Kellner in Austria. It found great industrial importance, but was largely abandoned in the 1970s for environmental reasons.
Since, in addition to sodium hydroxide and potassium hydroxide, chlorine was also always produced in a corresponding proportion, the chemical industry was interested early on in finding market applications for the abundant chlorine. During the First World War , part of the chlorine was used to produce war gases. Between 1950 and 1960, many toxic and poorly biodegradable organic chlorine compounds such as PCB or DDT were produced.
In 1963, Henri Bernard Beer developed the dimensionally stable, oxide-coated titanium anode for the diaphragm process.
In the 1970s, the more modern membrane process was developed in Japan in response to Minamata / Niigata disease , which was caused by chronic mercury poisoning.
Basics
The salt to be processed is dissolved in water and supplied as "brine":
Due to the autoprotolysis of the water, H 3 O + ( oxonium cations) and OH - ( hydroxide anions ) are always formed in the solution:
If you apply a voltage to the electrodes , work is done on the cell. It is an electrolytic cell . The ions which have the lowest decomposition potential are preferably oxidized . This can be explained thermodynamically with the free enthalpy :
where F is Faraday's constant , the number of electrons transferred and the standard reduction potential of the reaction. If the value of the free enthalpy is less than zero, the reaction takes place voluntarily (exergonic reaction). If the value is greater than zero, it does not take place voluntarily (endergonic reaction). For chlorine, a conventional reaction (as a reduction equation) results in a standard potential of +1.36 volts.
but since an oxidation should take place and not a reduction, the sign of the standard potential of the reaction is reversed:
this results in the free enthalpy:
- .
The oxidation takes place involuntarily , it is an endergonic reaction : If the electrode materials are chosen appropriately, these are the chloride ions, which are oxidized, and the oxonium ions, which are reduced. What remains are the sodium and hydroxide ions that form the caustic soda.
- Cathodic reaction
Dissociation of water Cathodic reaction Overall reaction in the cathode compartment
- Anode reaction
Anode reaction Overall reaction in the anode compartment
- Overall response
For the technical implementation it is important that the chlorine produced does not come into contact with the hydroxide ions, which would disproportionate to chloride and hypochlorite and contaminate the product:
In addition, care must be taken to ensure that the chlorine gas does not mix with the hydrogen gas, as this would create explosive chlorine gas . All of the following procedures meet these requirements.
Technical procedures
Diaphragm process
In the diaphragm process, the cathode consists of steel or steel coated with nickel, the anode consists of titanium coated with ruthenium (IV) oxide . The cathode compartment is separated from the anode compartment by a porous partition that is permeable to electricity and cations , so that the chlorine formed on the anode does not come into contact with either the hydrogen formed on the cathode or the hydroxide ions that are also formed . Otherwise chlorine would disproportionate with the OH - ions to Cl - and OCl - .
The redox couple H 2 / H 3 O + has a higher potential than Na / Na + , and the overvoltage of the hydrogen on iron is not very great, so hydrogen and not sodium develops on the cathode . Due to the discharge of the oxonium ions at the cathode, the solution in the cathode compartment is basic. The be due to the overvoltage at the anode oxygen on titanium , the Cl - discharged ions.
Anode:
Cathode:
The diaphragm is usually made of asbestos . Today, diaphragms made of polytetrafluoroethylene and inorganic additives are also used. Since the diaphragm cannot completely prevent the hydroxide ions present in the solution from penetrating into the anode space, a reaction to water and oxygen is possible if the hydroxide ion concentration is increased . Therefore, only a sodium hydroxide solution up to a concentration of about 12-15% can be obtained.
In EU parlance, the use of asbestos is not the best technology available .
Membrane process
The membrane process works with a titanium anode and a nickel cathode. The decisive difference to the diaphragm process is that in the membrane process, a 0.1 mm thick chlorine-resistant cation exchange membrane, which consists of polytetrafluoroethylene (PTFE / Teflon) with negatively charged SO 3 residues ( Nafion ), replaces the diaphragm. The anions such as OH - or Cl - cannot pass them, whereas the positively charged Na + ions do. Due to the impermeability to Cl - ions, a 30-33% sodium hydroxide solution is produced that is barely contaminated by sodium chloride. The chemical processes on the electrodes correspond to those in the diaphragm process.
Anode (plus pole):
Cathode (minus pole):
This process, which is the newest of the one presented here, is used nowadays in around 2/3 of the large-scale operations, since the end products Cl 2 , H 2 and NaOH are almost the same purity as in the amalgam process, but overall a significantly lower level Use of energy is required. Furthermore, the use of mercury, which is controversial from an environmental point of view, can be completely dispensed with.
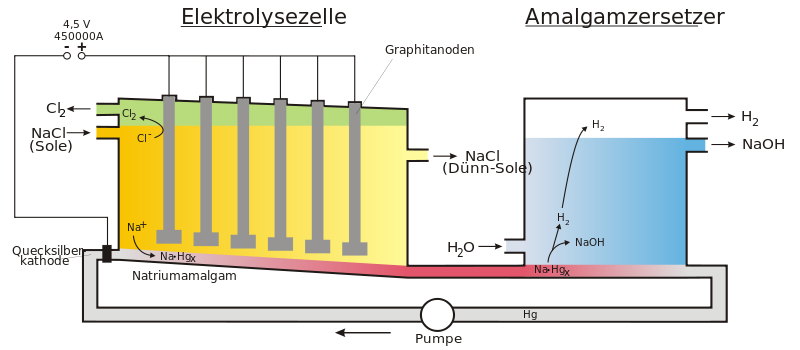
Amalgam process
In the amalgam process, the electrolysis of sodium chloride solution takes place between a titanium anode and the eponymous mercury cathode. Graphite anodes were used until the early 1970s, but they had a significantly shorter service life than modern titanium anodes. Chlorine gas is deposited at the anode . The sodium formed at the cathode immediately dissolves in the mercury as sodium amalgam . The amalgam is then reacted with water to graphite - catalysis decomposed, followed by sodium hydroxide and hydrogen are formed. The remaining mercury is fed back into the process.
The deposition of chlorine and sodium on the electrodes is based on the shift in the deposition potential of the elements hydrogen and oxygen due to overvoltages .
Theoretically, the following electrode reactions could take place:
Anode reaction ( oxidation ):
Cathode reaction ( reduction ):
Accordingly, hydrogen and oxygen would have to be separated. However, by choosing the suitable electrode material (titanium anode and mercury cathode) and the correct concentration ratios, sodium and chlorine are separated.
The sodium reacts immediately at the mercury cathode to form sodium amalgam:
To obtain caustic soda, the sodium amalgam is reacted with water in the amalgam decomposer. Decomposition reaction:
Overall response:
Due to the mercury emissions and the high power consumption, the amalgam process is increasingly being replaced by the membrane process worldwide. Under no circumstances is the amalgam process considered the best available technique in the European Union . According to the Minamata Convention , the use of the amalgam process is to be phased out worldwide by 2025, with exceptions being possible under certain conditions.
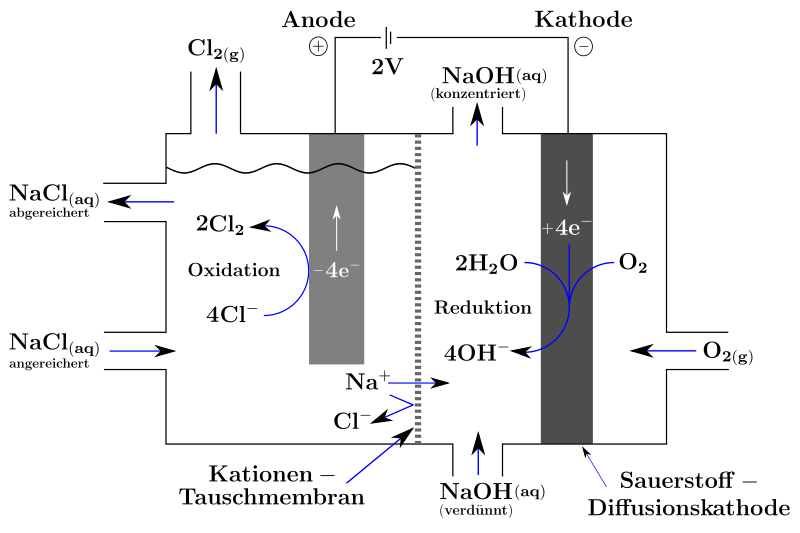
Oxygen Depletion Cathode (SVK)
The oxygen depolarized cathode ( SVK , ODC for " oxygen depolarized cathodes ") was developed around the turn of the millennium by Bayer MaterialScience , now Covestro, and has since been sold internationally by ThyssenKrupp Uhde / Uhdenora. The process is characterized above all by the reduced power consumption. The project is also funded by the Federal Ministry of Education and Research .
In chlorine electrolysis with an oxygen-consuming cathode, the same electrolysis cell is used as in the membrane process, except that the conventional cathode is replaced by one with a special oxygen diffusion surface. Oxygen is introduced behind this. The oxygen is reduced to hydroxide ions together with water:
Cathode ( reduction ):
Anode ( oxidation ):
Overall reaction:
The hydroxide ions of the caustic soda now come from oxygen reduced with water and no longer from the autoprotolysis of the water. As a result, protons are no longer reduced to hydrogen gas. This allows the operating voltage of the cell to be reduced significantly: Instead of 3 volts, only 2 volts are required. It is true that the by-product hydrogen is lost, but it can be produced more efficiently by direct chemical means than by electrolysis, since the typical conversion losses of 50 to 65% of the initial energy are avoided.
With up to 450,000 A per cell, the chlorine electrolysis requires considerable amounts of electricity. Due to the reduced operating voltage, enormous energy savings are possible with the oxygen-consuming cathode. According to Tony Van Osselaer (former board member of Bayer MaterialsScience), Germany's electricity needs could be reduced by 1% if all companies involved in the synthesis of chlor-alkali were to switch to SVK technology. Since this involves investments, the conversion from older technologies is still being postponed in many places. The SVK is a very good example of the further development of a process, the development of which has actually already been assessed as "completed".
The chloride ions can also come from sources other than sodium chloride (table salt), e.g. B. from hydrochloric acid as a waste product from the production of polyurethanes.
Comparison of the methods
Since the chlor-alkali electrolysis requires a lot of electrical energy, it is very expensive. In addition, companies that are active in this industry must also think about environmental impact. The state of the art must also be used to find out which process is the best available technology .
| method | advantages | disadvantage |
|---|---|---|
| Diaphragm process |
|
|
| Membrane process |
|
|
| Amalgam process |
|
|
| Oxygen depleting cathode |
|
|
World production and shares in the process
In 2012, the worldwide production capacity of chlorine was around 77 million tons per year. China had the largest share of this with around 40%; the European Union had a share of around 16%. Chlorine production in the European Union amounted to around 9.5 million t in 2013, Germany has a share of around 40%. The largest European manufacturer with a share of almost 20% is Dow Chemical in Stade and Schkopau .
The share of the manufacturing processes worldwide in 1990 was 39% amalgam, 45% diaphragm and 16% membrane processes. In the United States, the diaphragm process was traditionally the most prevalent (67% market share in 2003), in Western Europe the amalgam process and in Japan, due to its early introduction there, the membrane process. In 2005, the membrane process was used exclusively in Japan.
In the EU, the share of the membrane process has meanwhile increased to over 60% (2014).
See also
literature
- FR Minz, R. Schliebs: Modern processes in large-scale chemistry: chlorine and caustic soda. In: Chemistry in Our Time . 12th year 1978, No. 5, pp. 135-145.
- Peter Schmittinger (Ed.): Chlorine: Principles & Industrial Practice. Wiley, 2008, ISBN 978-3-527-61339-7 .
Web links
on best available techniques in the production of chlor-alkali
on production, capacities and locations of chlorine production in the European Union:
- Euro Chlor: Chlor-Alkali-Industry Review 2018–2019
swell
- ↑ a b c d e Thomas F. O'Brien: History of the Chlor-Alkali Industry (2005)
- ↑ Wolfgang Metternich, From the early days of the chemical industry to Griesheim industrial park: 150 years of chemistry in Griesheim , Festschrift, Griesheim industrial park, Frankfurt am Main 2006.
- ↑ Entry on ruthenium. In: Römpp Online . Georg Thieme Verlag, accessed April 10, 2011.
- ↑ a b New process reduces energy consumption in chlorine production by 30%. In: Process . June 14, 2013, accessed June 2, 2016 .
- ↑ GDCH weekly newsreel: " Saving energy through efficiency - success in chemistry"
- ↑ Efficient green gas - chemietechnik.de. In: chemietechnik.de. Retrieved June 2, 2016 .
- ↑ Imad Moussallem, Jakob Jörissen, Ulrich Kunz, Stefan Pinnow, Thomas Turek: Chlor-alkali electrolysis with oxygen depolarized cathodes: history, present status and future prospects . In: Journal of Applied Electrochemistry . tape 38 , no. 9 , May 14, 2008, p. 1177–1194 , doi : 10.1007 / s10800-008-9556-9 .
- ↑ a b bayer.de: Oxygen- consuming cathode (link is dead)
- ↑ Bayer receives environmental award for oxygen depolarized cathode technology. In: Process . June 24, 2008, accessed June 2, 2016 .
-
↑ "Reduction of Hg emissions by over a power of ten down to 20 g per ton of chlorine in 1979" (Holleman-Wiberg)
today: 0.68 g Hg / t chlorine (2013, Eurochlor) - ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 436.