Epigallocatechin gallate
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
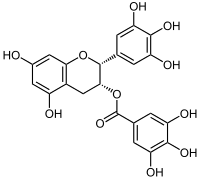
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Epigallocatechin gallate | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 22 H 18 O 11 | |||||||||||||||||||||
| Brief description |
white solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 458.36 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
217 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Epigallocatechin gallate , English. Epigallocatechin gallate , ( EGCG ), is a carboxylic acid ester of gallic acid with the alcohol and catechin epigallocatechin . The antioxidant makes up about a third of the dry mass of green tea . The proportion of catechins in black tea is significantly lower, as the catechins react to oligomeric theaflavins due to fermentation . The compound is said to have a positive effect on health.
Occurrence
tea
It is found in relatively high quantities in teas that contain caffeine, most of them in unfermented tea, see green tea .
Other sources
Traces can also be found in the skin of apples (especially the old apple varieties containing polyphenols ), in plums , onions , hazelnuts , pecans , pistachios , pears , kiwis , raspberries , strawberries . Comparatively much more is found in the fruit powder and the flour made from the seeds of the carob tree .
Medical aspects
Angiogenesis
Various experiments show that EGCG has an antiangiogenic effect (growth inhibition of blood vessels). This effect could be shown after xenotransplantation of pathological human endometrial tissue into mice. The human origin of the tissue suggests an effect in human endometriosis . In addition, similar effects have been demonstrated in in vitro and in vivo experiments with hamsters .
An antiangiogenic effect of EGCG could also be used to reduce the growth of tumor tissue. Tumors also need blood vessels to be adequately supplied with oxygen and nutrients. EGCG inhibits the activity of growth factors required in the mechanism of angiogenesis , such as B. the pro-angiogenic interleukin-8 . The binding of the peptide hormone VEGF to its receptor is inhibited by EGCG. Inhibition of VEGF-dependent angiogenesis by EGCG has been shown in a mouse model of gastric cancer.
A positive influence on the elasticity of the blood vessels (endothelial function), which plays a central role in the development of arteriosclerosis (atherogenesis), has also been reported.
Cell cycle
EGCG also supports the tumor suppressor protein P27 , which controls the cell cycle and prevents out of control cell division. It inhibits growth factors by binding to them.
Neurodegenerative Diseases
Neurodegenerative diseases like Alzheimer's and Parkinson's are caused by the formation of amyloid fibrils . EGCG prevents their formation by binding to the native, still unfolded polypeptide chains. As a result, harmless, spherical oligomers are formed instead of the toxic, fibrous amyloid fibrils . Studies indicate that EGCG can dissolve plaques. In the model organism color mouse it could be shown that after six months of EGCG treatment the plaque load in the cortex, hippocampus and in the entorhinal cortex was reduced by 54, 43 and 58% respectively.
immunology
A Japanese research group showed in laboratory tests that EGCG can prevent the invasion of HI viruses in T lymphocytes because, like HI viruses, it has an affinity for the CD4 molecules on the cell surface of the T lymphocytes. By occupying the CD4 receptors by the EGCG, the virus can no longer dock onto the T lymphocytes and thus no longer infect them.
EGCG was also shown to be effective against the influenza A virus. In experiments with cell cultures as well as in animal experiments, it was able to significantly reduce the replication of the virus and significantly increase the mean survival time in infected mice.
In addition, studies in multiple sclerosis research indicate that EGCG both protect nerve cells in the central nervous system from damage and regulate misdirected T lymphocytes, which are held responsible for the disease. The course of experimental autoimmune encephalomyelitis - the animal model of multiple sclerosis - was significantly milder when EGCG was administered than in animals that received no EGCG.
EGCG also neutralizes TNF-α and thus reduces the production of interleukin-6 and -8 , which explains the partly immunosuppressive effect.
metabolism
Catechins are generally considered to be radical scavengers of reactive oxygen (ROS) and nitrogen species (RNS). These two types of compounds are responsible for oxidative damage to DNA.
Binding to the cannabinoid receptor 1
EGCG binds to cannabinoid receptor 1 with a binding affinity of K i = 33.6 μM.
Potent inhibition of 11 β -hydroxysteroid dehydrogenase 1
A study from 2014 found that epigallocatechin gallate of green tea potently inhibits 11 β -hydroxysteroid dehydrogenase 1 , which means that less cortisol is available and thus some of the health-promoting properties of green tea could possibly be explained, as cortisol plays a decisive role in the psychophysiological stress response plays.
Heat stability and epimerization
In a high-temperature environment, epimerization of (-) - gallocatechin gallate to (-) - EGCG and decomposition is possible. Exposure to boiling water for 30 minutes results in a 12.4% reduction in the total amount of EGCG. With the short brewing time of tea, a reduction in EGCG is therefore insignificant.
Interaction with milk proteins
Various studies indicate that the proteins contained in milk ( whey proteins , caseins ) react with EGCG and other catechins. A reduction in the (-) - EGCG content in green tea when milk was added was observed.
possible side effects
From a daily dose of 800 mg EGCG, liver damage and increased blood pressure are to be feared. However, these only occurred when EGCG was administered as a dietary supplement or as a drug tested in controlled studies. Such effects are not observed even through extensive consumption of green tea in infusion form, which is why the European Food Safety Authority, EFSA, considers this form of consumption to be harmless to health in contrast to administration as a dietary supplement.
Web links
- Federal Ministry of Education and Research : Green tea against multiple sclerosis.
- Werner Hunstein : AL amyloidosis and EpiGalloCatechin-3-gallate: 4 years and 5 months later. Status: February 2011
literature
- H. Tachibana: Green tea polyphenol sensing. In: Proceedings of the Japan Academy. Volume 87, Number 3, 2011, pp. 66-80, PMID 21422740 . PMC 3066547 (free full text). (Review).
- D. Chen, SB Wan et al .: EGCG, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment. In: Advances in clinical chemistry. Volume 53, 2011, pp. 155-177, PMID 21404918 . (Review).
- N. Khan, VM Adhami, H. Mukhtar: Review: green tea polyphenols in chemoprevention of prostate cancer: preclinical and clinical studies. In: Nutrition and Cancer . Volume 61, number 6, 2009, pp. 836-841, doi: 10.1080 / 01635580903285056 . PMID 20155624 . PMC 2991093 (free full text). (Review).
- Hirofumi Tachibana, Kiyoshi Koga, Yoshinori Fujimura, Koji Yamada: A receptor for green tea polyphenol EGCG. In: Nature Structural & Molecular Biology . April 2004, pp. 380-381, doi: 10.1038 / nsmb743 . PMID 15024383 .
- Werner Hunstein: Epigallocathechin-3-gallate in AL amyloidosis: a new therapeutic option? In: Blood . Volume 110, number 6, September 2007, p. 2216, doi: 10.1182 / blood-2007-05-089243 . PMID 17785589 .
Individual evidence
- ↑ Qianying Dai, Yuanyuan He et al. a .: Effect of interaction of epigallocatechin gallate and flavonols on color alteration of simulative green tea infusion after thermal treatment. In: Journal of Food Science and Technology. 54, 2017, p. 2919, doi : 10.1007 / s13197-017-2730-5 .
- ↑ Entry on epigallocatechin-3-gallate. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ↑ a b data sheet (-) - Epigallocatechin gallate from Sigma-Aldrich , accessed on May 27, 2017 ( PDF ).
- Jump up ↑ Y. Zuo, H. Chen, Y. Deng: Simultaneous determination of catechins, caffeine and gallic acids in green, Oolong, black and pu-erh teas using HPLC with a photodiode array detector. In: Talanta. Volume 57, Number 2, May 2002, pp. 307-316, PMID 18968631 .
- ↑ a b Bhagwat, Seema; Haytowitz, David B .; Holden, Joanne M. (September 2011) .: USDA Database for the Flavonoid Content of Selected Foods, Release 3 (PDF) (Report). (PDF) Agricultural Research Service, US Department of Agriculture, September 2011, accessed on May 18, 2015 .
- ↑ ndr.de: Healthy old apple varieties - also for allergy sufferers , September 9, 2014.
- ↑ a b H. Xu, CM Becker et al: Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C / vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. In: Fertility and Sterility . Volume 96, number 4, October 2011, pp. 1021-1028.e1, doi: 10.1016 / j.fertnstert.2011.07.008 . PMID 21821246 .
- ↑ MW Laschke, C. Schwender et al: Epigallocatechin-3-gallate inhibits estrogen-induced activation of endometrial cells in vitro and causes regression of endometriotic lesions in vivo. In: Human Reproduction . Volume 23, Number 10, October 2008, pp. 2308-2318, doi: 10.1093 / humrep / den245 . PMID 18603648 .
- ↑ a b H. Y. Shin, SH Kim, HJ Jeong, SY Kim, TY Shin, JY Um, SH Hong, HM Kim: Epigallocatechin-3-gallate inhibits secretion of TNF-alpha, IL-6 and IL-8 through the attenuation of ERK and NF-kappaB in HMC-1 cells . In: International Archives of Allergy and Immunology . November 2006, PMID 17135765 .
- ↑ Takako Kondo, Toshiro Ohta, Koichi Igura, Yukihiko Hara, Kazuhiko Kaji: Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding . In: Cancer Letters . tape 180 , no. 2 , 2002, p. 139-144 , doi : 10.1016 / S0304-3835 (02) 00007-1 .
- ^ BH Zhu, WH Zhan, ZR Li, Z. Wang, YL He, JS Peng, SR Cai, JP Ma, CH Zhang: (-) - Epigallocatechin-3-gallate inhibits growth of gastric cancer by reducing VEGF production and angiogenesis. In: World journal of gastroenterology: WJG. Volume 13, Number 8, February 2007, pp. 1162-1169. PMID 17451194 .
- ↑ ME Widlansky, NM Hamburg et al .: Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. In: Journal of the American College of Nutrition. Volume 26, Number 2, April 2007, pp. 95-102. PMID 17536120 .
- ↑ M. Lorenz, S. Wessler, E. Follmann, W. Michaelis, T. Düsterhöft, G. Baumann, K. Stangl, V. Stangl: A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation . In: Journal of Biological Chemistry . February 2004, PMID 14645258 .
- ↑ M. Nihal, CT Roelke, GS Wood: Anti-melanoma effects of vorinostat in combination with polyphenolic antioxidant ( -) - epigallocatechin-3-gallate (EGCG) . In: Pharmaceutical Research . June 2010, PMID 20232120 .
- ↑ a b D. E. Ehrnhoefer, J. Bieschke, A. Boeddrich, M. Herbst, L. Masino, R. Lurz, S. Engemann, A. Pastore, EE Wanker: EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers . In: Nature Structural & Molecular Biology . June 2008, PMID 18511942 .
- ↑ K. Rezai-Zadeh, GW Arendash et al: Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice . In: Brain Research . tape 12 , no. 1214 , June 2008, p. 177-186 , PMID 18457818 .
- ↑ F. Jiang, W. Chen, K. Yi, Z. Wu, Y. Si, W. Han, Y. Zhao: The evaluation of catechins that contain a galloyl moiety as potential HIV-1 integrase inhibitors . In: Clin Immunol. December 2010, PMID 20832370 .
- ↑ X. Xiao, ZQ Yang, LQ Shi, J. Liu, W. Chen: [Antiviral effect of epigallocatechin gallate (EGCG) on influenza A virus]. In: Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica. Volume 33, Number 22, November 2008, pp. 2678-2682. PMID 19216171 .
- ^ O. Aktas, T. Prozorovski, A. Smorodchenko, NE Savaskan, R. Lauster, PM Kloetzel, C. Infante-Duarte, S. Brocke, F. Zipp: Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis . In: Journal of Immunology . November 2004, PMID 15494532 .
- ↑ Does green tea slow down inflammatory processes in the central nervous system? Article from the German Multiple Sclerosis Society .
- ↑ KW Lee, HJ Lee: The roles of polyphenols in cancer chemoprevention. In: BioFactors. Volume 26, Number 2, 2006, pp. 105-121, PMID 16823097 . (Review).
- ↑ G. Korte, A. Dreiseitel, P. Schreier, A. Oehme, S. Locher, S. Geiger, J. Heilmann, PG Sand: Tea catechins' affinity for human cannabinoid receptors. In: Phytomedicine . Volume 17, number 1, January 2010, pp. 19-22, doi: 10.1016 / j.phymed.2009.10.001 . PMID 19897346 .
- ↑ Jan Hintzpeter, Claudia Stapelfeld, Christine Loerz, Hans-Joerg Martin, Edmund Maser: Green Tea and One of Its Constituents, Epigallocatechine-3-gallate, Are Potent Inhibitors of Human 11β-hydroxysteroid Dehydrogenase Type 1. In: PLOS ONE . 9, 2014, p. E84468, doi: 10.1371 / journal.pone.0084468 .
- ^ R. Wang, W. Zhou, X. Jiang: Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. In: Journal of Agricultural and Food Chemistry . Volume 56, Number 8, April 2008, pp. 2694-2701, doi: 10.1021 / jf0730338 . PMID 18361498 .
- ↑ Imed Hasni, Philippe Bourassa, Saber Hamdani, Guy Samson et al: Interaction of milk α- and β-caseins with tea polyphenols . In: Food Chemistry . tape 126 , no. 2 , May 2011, p. 630-639 , doi : 10.1016 / j.foodchem.2010.11.087 .
- ↑ CD Kanakis, Imed Hasni et al: Milk β-lactoglobulin complexes with tea polyphenols . In: Food Chemistry . tape 127 , no. 3 , August 2011, p. 1046-1055 , doi : 10.1016 / j.foodchem.2011.01.079 .
- ↑ Elisabeth Jöbstl, Jonathan R. Howse et al.: Noncovalent Cross-Linking of Casein by Epigallocatechin Gallate Characterized by Single Molecule Force Microscopy. In: J. Agric. Food Chem. 54 (12), 2006, pp. 4077-4081 doi: 10.1021 / jf053259f
- ↑ F. Catterall, AI Kassimi, MN Clifford, C. Ioannides: Influence of milk on the antimutagenic potential of green and black teas. In: Anticancer Research . 23 (5A), Sep-Oct 2003, pp. 3863-3867. PMID 14666689
- ↑ EFSA assesses the safety of green tea catechins. April 18, 2018, accessed April 20, 2019 .
- ↑ Jesus Lovera, Alexander Ramos, Deidre Devier, Virginia Garrison, Blake Kovner: Polyphenon E, non-futile at neuroprotection in multiple sclerosis but unpredictably hepatotoxic: Phase I single group and phase II randomized placebo-controlled studies . In: Journal of the Neurological Sciences . tape 358 , no. 1-2 , November 2015, pp. 46–52 , doi : 10.1016 / j.jns.2015.08.006 ( elsevier.com [accessed April 20, 2019]).