Etilefrine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
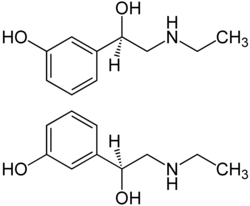
|
||||||||||||||||||||||
| 1: 1 stereoisomer mixture of ( R ) -enantiomer (top) and ( S ) -enantiomer (bottom) | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Etilefrine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 181.23 g · mol -1 (etilefrine) | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Etilefrine is a drug that is used for circulatory disorders associated with low blood pressure ( hypotension ), dizziness , unexplained tiredness , weakness, fibrillation and turning black in the eyes. Etilefrine is used as a mixture of enantiomers ( racemate ) in the form of the hydrochloride .
Mechanism of action
The substance is a direct sympathomimetic with α- and β-sympathomimetic effects. The α-adrenergic effect on the α- adrenoceptors on the blood vessels leads to vasoconstriction and thus to an increase in blood pressure lasting several hours. Since at the same time there is a β-adrenergic effect, which results from the influence on the β-adrenoceptors of the heart , there is also an increase in the beat frequency ( pulse ) and the beat strength ( inotropy ) of the heart. The half-life ( elimination ) is approx. 2 hours.
Side effects (selection)
- Palpitations
- ventricular arrhythmias
- Angina pectoris -like complaints
- Micturition disorders due to stimulation of the bladder sphincter (disorders when emptying the urinary bladder )
- Restlessness
- increased sweating
- insomnia
Contraindications (selection)
- Hyperthyroidism (abnormal overactive thyroid gland )
- Pheochromocytoma (tumor that produces noradrenaline , adrenaline, and metanephrine )
- benign prostatic hyperplasia with residual urine formation (benign enlargement of the prostate )
- Cardiac arrhythmia
- Valve stenosis (narrowing of the heart valves )
- Cardiomyopathy (diseases of the heart muscle )
doping
Etilefrin is listed on the World Anti-Doping Agency (WADA) list as a stimulant that is banned during athletic competitions.
Trade names
Bioflutin (D), Effortil (D, A, CH), Pholdyston (D), Thomasin (D), Etil (D), Etilefrin (D)
Etilefrine in combination with dihydroergotamine in liquid form as Effortil plus (CH) (out of trade)
Web links
- Entry on Etilefrin at Vetpharm, accessed on January 10, 2012.
Individual evidence
- ↑ a b Entry on etilefrine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, pp. 658-659, ISBN 978-0-911910-00-1 .
- ↑ a b Entry on Etilefrine Hydrochloride at TCI Europe, accessed on January 10, 2012.
- ↑ a b Mutschler: drug effects. 8th edition.
- ↑ Red List Online
- ↑ Entry on etilefrine. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ^ The 2009 Prohibited List, International Standard . ( Memento from July 19, 2014 in the Internet Archive ; PDF; 177 kB) The World Anti-Doping Code, p. 7.
- ↑ Entry on Etilefrin in Pharmawiki , accessed on July 25, 2016.