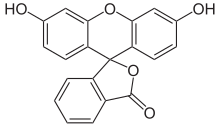
Fluorescein
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Fluorescein | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 20 H 12 O 5 | |||||||||||||||||||||
| Brief description |
red crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 332.32 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
314–316 ° C (decomposition) |
|||||||||||||||||||||
| solubility |
almost insoluble in water, easily soluble in ethanol , DMSO , diethyl ether and alkalis |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Fluorescein is a fluorescent dye from the group of xanthene dyes and triphenylmethane dyes .
history
Fluorescein and other phthaleins were discovered by Adolf von Baeyer in 1871 . The substance owes its alternative name resorcinphthalein to the reactants from which Adolf von Baeyer synthesized it. He mixed phthalic anhydride (phthalic acid can also be used) with resorcinol and added concentrated sulfuric acid as a dehydrating agent. Then he heated the mixture to a viscous melt and then treated the cooled melt with warm water and ammonia , then he brought the batch into plenty of distilled water.
presentation
Fluorescein ( 2 ) is usually produced by condensation of one equivalent of phthalic anhydride ( 1 ) and two equivalents of resorcinol with elimination of water on a catalyst . Either concentrated sulfuric acid or anhydrous zinc chloride is suitable as a catalyst .
properties
Fluorescein comes in two different structures. The more stable is the open carboxylic acid form, which is present in the solid state as red crystals. There is also the spiro- lactone form, which forms an unstable, yellow compound.
- Absorption maximum: 485 nm (pH 9)
- Extinction coefficient : 93 000 l cm −1 mol −1 (pH 9)
- Emission maximum: 514 nm (pH 9)
- Quantum yield : 0.93 (50 µmol fluorescein in 0.1 mol sodium borate buffer, pH 9.5)
- Fluorescence lifetime : 4.16 ns (dianion in sodium hydroxide)
use
Fluorescein is used as an indicator in analytical chemistry . In addition, it is used to detect bromides , to color sources and to color soaps and bath extracts / salts. Furthermore, fluorescein is a fluorescent dye which emits green light (wavelength approx. 520 to 530 nm) when excited with blue light ( absorption maximum at a wavelength of 496 nm, e.g. by an argon ion laser ). Related reactive dyes such. B. Fluorescein isothiocyanate (FITC) can be coupled with various antibodies (immunoglobulins) and thus used for fluorescence microscopy or flow cytometry . In this way, specific surface properties ( antigens ), e.g. B. also of pathogens can be detected. This working technique is called direct immunofluorescence . Furthermore, fluorescein is used as a staining agent in ophthalmology for fluorescein angiography and applanation tonometry .
Fluorescein has a high quantum yield of up to 93%, but its usefulness for fluorescent labeling is limited by a number of disadvantages: The intensity of the fluorescence depends on the pH value and falls well below pH 7 (pK s ≈ 6.4). The fluorescence also decreases rapidly under illumination (strong photo-bleaching ). The fluorescence maximum is not very sharp, the associated absorption band is quite broad.
Analytics

The classic detection is based on the bromination through which the red eosin (tetrabromofluorescein) is formed. In addition, dilute solutions of fluorescein stand out due to their yellow-green fluorescent color, on which the name is based.
Uranine
The water-soluble sodium salt of fluorescein bears the name uranin (same CI number 45350, empirical formula C 20 H 10 Na 2 O 5 ) and is a widely used yellow dye that fluoresces green under UV and daylight .
Uranine has an enormous coloring power in aqueous solution (against a white background, a uranium concentration of 0.1 mg / l leads to a visible discoloration).
In the case of shipwrecked people or after ditching, a sea surface of approx. 4000 m² can be conspicuously colored with a quantity of just 500 g uranine and thus discovery by search parties, especially from the air, can be made easier.
The dye is also used in a variety of ways, especially for coloring foam baths , bath additives, shampoos , cosmetics and antifreeze for car radiators, in order to be able to easily locate leaks in the cooling system using a UV lamp. If the seal between the cooling system and the combustion chamber is not tight, the dye can even be detected on the exhaust. It is also used for leak tests on flat roofs and tank systems. Uranine is considered biologically harmless. It is therefore used by hydrologists and hydrogeologists as a so-called tracer to track groundwater flows and underground rivers. In 1877 10 kg uranine was introduced into the Danube near Immendingen and 60 hours later a clear fluorescence was noticed in the river Aach , which could explain the sinking of the Danube .
Uranine is also used for decoration, for example for luminous colors in discotheques or scene sets (in science fiction or horror films: Uranine irradiated with UV light looks “toxic-radioactive”). Annually on March 17th, St. Patrick's Day , the Chicago River that flows through Chicago was colored green with uranine. Due to the intervention of the EPA , uranine has been replaced by another dye since 1966 or 2003. Uranine was used by environmental activists on September 10, 2019 to color the Limmat in Zurich green, which, however, led to a police investigation into water pollution.
Web links
Individual evidence
- ↑ a b c d e entry on fluorescein. In: Römpp Online . Georg Thieme Verlag, accessed on March 3, 2014.
- ↑ a b Entry on fluorescein in the GESTIS substance database of the IFA , accessed on February 10, 2017(JavaScript required) .
- ^ Robert L. Stamper, Marc F. Lieberman, Michael V. Drake: Becker-Shaffer's Diagnosis and Therapy of the Glaucomas. 8th edition, Elsevier Health Sciences, 2009, ISBN 978-0-323-02394-8 , pp. 47-50.
- ↑ Always in flux: The perfect dye marking solution. In: Merck Millipore Homepage. Merck KGaA, accessed April 14, 2012 .
- ↑ Working group "Human and ecotoxicological evaluation of marking agents in waters": Human and ecotoxicological evaluation of marking agents in water. Recommendations of a working group at the Federal Environment Agency . In: groundwater . tape 2 , no. 2 , June 1, 1997, p. 61-64 , doi : 10.1007 / s767-1997-8521-7 .
- ↑ A. Knop: About the hydrographic relationships between the Danube and the Aach spring in the Baden Oberland. In: New Year Mineral. Geol. Palaeontol. , (1878), pp. 350-363.
- ↑ Miriam Krause: Celebrating St. Patrick's Day with Fluorescence . In: Sustainable nano, March 17, 2015.
- ^ Other cities dye-ing to know what turns Chicago river green. Reported in The Columbia Chronicle March 20, 2003.
- ↑ Environmental activists are coloring the Limmat green