Favipiravir
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
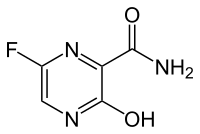
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Favipiravir | |||||||||||||||
| other names |
6-fluoro-3-hydroxy-2-pyrazine carboxamide |
|||||||||||||||
| Molecular formula | C 5 H 4 FN 3 O 2 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 157.1 g · mol -1 | |||||||||||||||
| Melting point |
176-178 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Favipiravir (synonymous T-705 ) is an antiviral drug that is used as Avigan for the oral treatment of infections with various RNA viruses . Like the structurally related antivirals T-1105 and T-1106, it belongs to the pyrazine carboxamides . Favipiravir was used in humans during the Ebola virus epidemic in 2014 without the usually necessary drug approval.In May 2020 it received approval as avifavir in Russia for the treatment of COVID-19 .
Presentation and extraction
Various synthetic routes are known for the production of favipiravir. One synthesis variant starts from 3-hydroxypyrazine-2-carboxylic acid. The 3-hydroxypyrazine-2-carbonamide is first obtained via the acid chloride by reaction with thionyl chloride and subsequent reaction with aqueous ammonia . Nitration with potassium nitrate and sulfuric acid results in nitro group substitution in the 6-position. In the following step, this is reduced to the amine using hydrazine hydrate , which is diazotized as an intermediate using sodium nitrite and converted to the target compound with the hydrogen fluoride-pyridine complex.
properties
Favipiravir is guanine - analogue and an inhibitor of the viral RNA-dependent RNA polymerase by different viruses , but not by cellular polymerases . It also increases the mutation rate in the replication of the influenza virus and the Ebola virus . Favipiravir is a prodrug , which means that it is metabolized by the HGPRT into favipiravir -ribofuranosyl-5'-monophosphate (FRMP) and favipiravir-ribofuranosyl-5'-triphosphate (FRTP), with FRTP being the active form of favipiravir Inhibition of RNA-dependent RNA polymerase is.
Favipiravir is effective against influenza virus, foot-and-mouth disease virus , various flaviviruses (the West Nile virus , the yellow fever virus ), arenaviruses , bunyaviruses and alphaviruses , some enteroviruses , the nipah virus , noroviruses , the ebola virus , among others , Lassa virus , rabies virus and Rift Valley fever virus. It also works against the Zika virus , but worse than MK-608.
Therapeutic and experimental use
Favipiravir was developed by Toyama Kagaku Kōgyō , a subsidiary of Fujifilm Holdings , and patented in 1999. Favipiravir was originally developed in Japan as an influenza drug (trade name: Avigan ), whereby drug approval was limited to the treatment of influenza with novel virus strains that do not respond to common antivirals; Because of the teratogenic ( teratogenic ) effects observed in many species , it is also contraindicated in pregnancy. During the 2014 Ebola fever epidemic, Japan offered the WHO to supply favipiravir on demand. On October 4, 2014, a French nurse from Médecins Sans Frontières was treated with favipiravir and survived an Ebola virus infection that she contracted in Liberia. The World Health Organization wrote in a statement that in the course of the Ebola virus epidemic in 2014, it was ethically acceptable to use preventive or therapeutic drugs without proof of effectiveness in humans, if promising results could be shown in animal experiments.
In February 2020, favipiravir was tested in China in a first non-randomized double-blind study on 80 patients as an antiviral therapy against the coronavirus SARS-CoV-2 . In another study in which favipiravir was compared to the virostatic preparation Arbidol ( umifenovir ) in around 120 patients each, favipiravir showed a significant improvement. Around 71 percent of moderately ill patients who were given favipiravir had recovered - sometimes with severe side effects - after seven days. With the Arbidol used in comparison , it was almost 56 percent. In a smaller group of seriously ill patients at the start of therapy, there was no clear difference. However, the results of this study are questioned. Favipiravir was previously approved for clinical trials in China in February 2020 to evaluate its effectiveness in COVID-19.
Avigan ( favipiravir ) is one of the drugs for which the Federal Ministry of Health initiated central procurement for the treatment of infected and seriously ill COVID-19 patients in Germany in April 2020. Since Covid-19 therapy is an individual healing attempt without clinical proof of effectiveness, its use should primarily be considered on a patient-by-patient basis in severe forms.
On May 29, 2020, the Russian Ministry of Health approved a generic version of favipiravir under the drug name Avifavir for the treatment of COVID-19. The Russian Direct Investment Fund (RDIF) supported the development of Avifavir and said it was shown to be highly effective in the first phase of clinical trials.
literature
- EJ Mifsud, FG Hayden, AC Hurt: Antivirals targeting the polymerase complex of influenza viruses. In: Antiviral research. Volume 169, 09 2019, p. 104545, doi : 10.1016 / j.antiviral.2019.104545 , PMID 31247246 .
Web links
- Avifavir. Medum.ru. In: Russian Medicines Manual.
- Favipiravir. US National Library of Medicine. In: Drug Information Portal.
Individual evidence
- ↑ a b Guo, Qi; Xu, Mingshuo; Guo, Shuang; Zhu, Fuqiang; Xie, Yuanchao; Shen, Jingshan: The complete synthesis of favipiravir from 2-aminopyrazine in Chemical Papers 73 (2019) 1043-1051, doi : 10.1007 / s11696-018-0654-9 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c Y. Furuta, K. Takahashi, K. Shiraki, K. Sakamoto, DF Smee, DL Barnard, BB Gowen, JG Julander, JD Morrey: T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. In: Antiviral Research . Volume 82, Number 3, June 2009, pp. 95-102, doi : 10.1016 / j.antiviral.2009.02.198 , PMID 19428599 .
- ↑ Furuta Y, Takahashi K .: Nitrogenous heterocyclic carboxamide derivatives or salts thereof and antiviral agents containing both , WO 00/10569 [P]. 2001-04-07.
- ↑ Fangyuan Shi; Zongtao Li; Lingjin Kong; Yuanchao Xie; Tao Zhang; Wenfang Xu: Synthesis and crystal structure of 6-fluoro-3-hydroxypyrazine-2-carboxamide in Drug Discoveries & Therapeutics 8 (2014) 117-120, doi : 10.5582 / ddt.2014.01028 .
- ^ E. De Clercq: New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections. In: Chemistry, an Asian journal. Volume 14, Number 22, November 2019, pp. 3962–3968, doi : 10.1002 / asia.201900841 , PMID 31389664 .
- ↑ Z. Jin, LK Smith, VK Rajwanshi, B. Kim, J. Deval: The ambiguous base-pairing and high substrate efficiency of T-705 (Favipiravir) Ribofuranosyl 5'-triphosphate towards influenza A virus polymerase. In: PLOS ONE . Volume 8, number 7, 2013, p. E68347, doi : 10.1371 / journal.pone.0068347 , PMID 23874596 , PMC 3707847 (free full text).
- ↑ T. Baranovich, SS Wong, J. Armstrong, H. Marjuki, RJ Webby, RG Webster, EA Govorkova: T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. In: Journal of Virology . Volume 87, number 7, April 2013, pp. 3741-3751, doi : 10.1128 / JVI.02346-12 , PMID 23325689 , PMC 3624194 (free full text).
- ↑ J. Guedj, G. Piorkowski, F. Jacquot, V. Madelain, TH Nguyen, A. Rodallec, S. Gunther, C. Carbonnelle, F. Mentré, H. Raoul, X. de Lamballerie: Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques. In: PLoS medicine. Volume 15, number 3, 03 2018, p. E1002535, doi : 10.1371 / journal.pmed.1002535 , PMID 29584730 , PMC 5870946 (free full text).
- ↑ DF Smee, BL Hurst, H. Egawa, K. Takahashi, T. Kadota, Y. Furuta: Intracellular metabolism of favipiravir (T-705) in uninfected and influenza A (H5N1) virus-infected cells. In: The Journal of antimicrobial chemotherapy. Volume 64, number 4, October 2009, pp. 741-746, doi : 10.1093 / jac / dkp274 , PMID 19643775 , PMC 2740635 (free full text).
- ↑ L. Naesens, LW Guddat, DT Keough, AB van Kuilenburg, J. Meijer, J. Vande Voorde, J. Balzarini: Role of human hypoxanthine guanine phosphoribosyltransferase in activation of the antiviral agent T-705 (favipiravir). In: Molecular pharmacology. Volume 84, number 4, October 2013, pp. 615-629, doi : 10.1124 / mol.113.087247 , PMID 23907213 .
- ^ Y. Furuta, BB Gowen, K. Takahashi, K. Shiraki, DF Smee, DL Barnard: Favipiravir (T-705), a novel viral RNA polymerase inhibitor. In: Antiviral research. Volume 100, number 2, November 2013, pp. 446-454, doi : 10.1016 / j.antiviral.2013.09.015 , PMID 24084488 , PMC 3880838 (free full text).
- ↑ S. Banerjee, N. Gupta, P. Kodan, A. Mittal, Y. Ray, N. Nischal, M. Soneja, A. Biswas, N. Wig: Nipah virus disease: A rare and intractable disease. In: Intractable & rare diseases research. Volume 8, number 1, February 2019, pp. 1–8, doi : 10.5582 / irdr.2018.01130 , PMID 30881850 , PMC 6409114 (free full text).
- ↑ a b L. Delang, R. Abdelnabi, J. Neyts: Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. In: Antiviral research. Volume 153, 05 2018, pp. 85-94, doi : 10.1016 / j.antiviral.2018.03.003 , PMID 29524445 .
- ↑ SJ Smither, LS Eastaugh, JA Steward, M. Nelson, RP Lenk, MS Lever: Post-exposure efficacy of oral T-705 (favipiravir) against inhalational Ebola virus infection in a mouse model. In: Antiviral research. Volume 104, April 2014, pp. 153-155, doi : 10.1016 / j.antiviral.2014.01.012 . PMID 24462697 .
- ↑ K. Yamada, K. Noguchi, T. Komeno, Y. Furuta, A. Nishizono: Efficacy of Favipiravir (T-705) in Rabies Postexposure Prophylaxis. In: The Journal of Infectious Diseases . Volume 213, number 8, April 2016, pp. 1253-1261, doi : 10.1093 / infdis / jiv586 , PMID 26655300 , PMC 4799667 (free full text).
- ↑ J. Murphy, CD Sifri, R. Pruitt, M. Hornberger, D. Bonds, J. Blanton, J. Ellison, RE Cagnina, KB Enfield, M. Shiferaw, C. Gigante, E. Condori, K. Gruszynski, RM Wallace: Human Rabies - Virginia, 2017. In: MMWR. Morbidity and mortality weekly report. Volume 67, number 5152, January 2019, pp. 1410–1414, doi : 10.15585 / mmwr.mm675152a2 , PMID 30605446 , PMC 6334827 (free full text).
- ^ Caroline AL, Powell DS, Bethel LM, Oury TD, Reed DS, et al. (2014) Broad Spectrum Antiviral Activity of Favipiravir (T-705): Protection from Highly Lethal Inhalational Rift Valley Fever. PLoS Neglected Tropical Diseases 8 (4): e2790. doi : 10.1371 / journal.pntd.0002790 .
- ↑ N. Mumtaz, JJ van Kampen, CB Reusken, CA Boucher, MP Koopmans: Zika Virus: Where Is the Treatment? In: Current treatment options in infectious diseases. Volume 8, 2016, pp. 208-211, doi : 10.1007 / s40506-016-0083-7 , PMID 27547128 , PMC 4969322 (free full text).
- ↑ FG Hayden, N. Shindo: Influenza virus polymerase inhibitors in clinical development. In: Current opinion in infectious diseases. Volume 32, number 2, 04 2019, pp. 176-186, doi : 10.1097 / QCO.0000000000000532 , PMID 30724789 , PMC 6416007 (free full text).
- ↑ Ebola outbreak: Japan offers anti-influenza drug for treatment . CBC News Health. August 25, 2014. Retrieved January 15, 2015.
- ↑ French nurse cured of Ebola . RFI . 4th October 2014.
- ↑ WHO - Ethical considerations for use of unregistered interventions for Ebola virus disease . Retrieved October 8, 2014.
- ↑ L. Dong, S. Hu, J. Gao: Discovering drugs to treat coronavirus disease 2019 (COVID-19). In: Drug discoveries & therapeutics. Volume 14, number 1, 2020, pp. 58-60, doi : 10.5582 / ddt.2020.01012 , PMID 32147628 .
- ↑ Wang Xiaoyu: Drugs with potential against coronavirus in human trials. In: China Daily website . February 15, 2020, accessed on February 17, 2020 .
- ^ A b Anja Martini, Christian Drosten : Coronavirus Update. (PDF) Episode 22. In: ndr.de. Norddeutscher Rundfunk, March 26, 2020, p. 6 f. , accessed on March 27, 2020 .
- ↑ Kazuhiro Nogi: What can the flu drug Avigan, which the federal government is buying, do? In: spiegel.de. April 2, 2020, accessed April 2, 2020 .
- ↑ Zhang Yangfei: First antiviral drug approved to fight coronavirus. In: China Daily website . February 17, 2020, accessed on February 17, 2020 .
- ↑ Information from the institutions and authorities: BMG: Central procurement of pharmaceuticals for the treatment of serious cases of COVID-19 infected patients and distribution to pharmacies by the Bundeswehr , Drugs Commission of German Pharmacists (AMK), news of March 24, 2020.
- ↑ Avifavir Included in the List of Nationally Recommended Drugs for Treatment of COVID-19. In: www.gmpnews.net. June 6, 2020, accessed June 16, 2020 .
- ↑ RDIF and ChemRar deliver first batch of Avifavir antiviral drug to Russian hospitals. In: www.pharmiweb.com. June 12, 2020, accessed June 16, 2020 .
