Imatinib
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
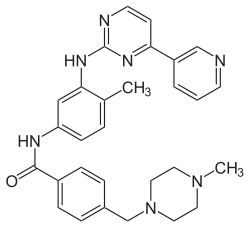
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Imatinib | |||||||||||||||
| other names |
4 - [(4-methylpiperazin-1-yl) methyl] - N - {4-methyl-3 - [(4-pyridin-3-ylpyrimidin-2-yl) amino] phenyl} benzamide |
|||||||||||||||
| Molecular formula | C 29 H 31 N 7 O | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| Mechanism of action | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 493.60 g mol −1 | |||||||||||||||
| Melting point |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Imatinib is a protein kinase inhibitor used in the treatment of chronic myeloid leukemia (CML), gastrointestinal stromal tumors (GIST) and other malignancies . It is the active ingredient of the drug sold by Novartis under the trade name Glivec (Europe / Australia) or Gleevec (USA). Imatinib mesilate, a salt of methanesulfonic acid , whose name was CGP57148B or STI-571 during the development phase, is used medicinally.
Mechanism of action
The Philadelphia chromosome is found in over 90% of patients with chronic myeloid leukemia (CML) . It is a shortened chromosome 22 , which is created by the exchange of genetic material between chromosome 9 and 22. This process is called translocation . This fuses the gene for a natural enzyme , tyrosine kinase ABL on chromosome 9, with a fragment of the BCR gene on chromosome 22. The cells mutated in this way produce a so-called BCR-ABL fusion protein, a tyrosine kinase with increased activity compared to ABL, which leads to the uncontrolled multiplication of white blood cells and plays a decisive role in the development of CML.
Imatinib is a specific inhibitor that blocks the activity of the tyrosine kinase ABL in the diseased cells and thus suppresses the pathologically increased proliferation of mutated blood stem cells.
The aim of treating chronic myeloid leukemia with imatinib is to reduce the number of pathological cell clones as far as possible. A molecular remission, in which BCR-ABL transcripts can no longer be detected even by means of sensitive methods (RT- PCR ), only reaches a small group of patients. About 40% achieve a significant reduction in BCR-ABL in the quantitative PCR (> 3 log levels), in at least 70% no metaphases with the Philadelphia chromosome can be detected (complete cytogenetic reduction). Normalization of the blood count (complete haematological remission) is achieved in more than 95% of patients with imatinib.
Molecular mechanism of action
The mechanism of action of Imatinib is the competitive and selective blockade of the ATP binding site of specific tyrosine kinases, such as. B. Abl, Bcr-Abl, c-Kit and the PDGF receptor. This blockage prevents the transfer of a phosphate residue to the substrate. Imatinib also acts on the physiologically occurring Abl. However, healthy cells have additional signaling pathways and their function is hardly disturbed. Cancer cells, on the other hand, depend on the activity of Bcr-Abl and are severely impaired in their ability to divide and survive. Imatinib also targets ARG, DDR1 and NQO2. The latter is an oxidoreductase and not a tyrosine kinase.
The tyrosine kinase inhibitors Dasatinib , Nilotinib and SGX393 are further developments of substances with similar mechanisms of action, which can represent a therapeutic alternative in the case of imatinib resistance or intolerance .
metabolism
| Bioavailability | 98% |
| Plasma protein binding | 95% |
| metabolism | Hepatic, predominantly cytochrome P450 3A4 |
| Half-life | 18-22 hours |
| excretion | Hepatic |
| Administration form | Orally |
| pregnancy | careful risk-benefit assessment ( teratogenic in animal experiments) |
Imatinib is mainly metabolized by the cytochrome P450 isoenzyme CYP3A4 , with the N-desmethyl piperazine derivative being the main metabolite with residual activity. Inhibitors of this isoenzyme ( e.g. erythromycin , cimetidine or grapefruit juice ) can inhibit the metabolism of imatinib and thus lead to an increase in plasma concentrations and consequently to higher toxicity. There are also N-oxides on the piperazine and pyrimidine rings, as well as the oxidation of the aromatic methyl group to alcohol . Most of it is excreted via the bile (biliary) and to a lesser extent via the kidneys . Unchanged, 25% are eliminated. Imatinib is a competitive inhibitor of the cytochromes CYP2C9 , CYP2D6, CYP3A4 / 5. The time of maximum plasma concentration is 1 to 2 hours.
Adverse effects and contraindications
Imatinib is generally well tolerated. The adverse effects include nausea, vomiting, diarrhea, muscle pain ( myalgia ), muscle cramps, reddening of the skin, increased liver values (increased transaminases ), swelling ( edema ) and skin changes. Imatinib is teratogenic in animal studies and should therefore not be used during pregnancy .
Physical Properties
The medicinally used mesilate is present as a crystalline solid, which can occur in two polymorphic crystal forms. The α-form melts at 220.5 ° C with a melting enthalpy of −22.28 J g −1 , the β form at 214.0 ° C with −31.38 J g −1 Both forms are enantiotropic to one another . At room temperature, the β-form is the thermodynamically stable form. A patent from 2013 describes four other polymorphic forms that have a higher solubility than the β form.
Patent law aspects
When the patent protection of the original preparation Glivec expires , generic manufacturers in European countries want to come onto the market with generic drugs . In January 2013, Imatinib Teva ( Teva ) was approved for the indication of chronic myelogenous leukemia (CML) in Philadelphia chromosome- positive children and adults ; Imatinib Actavis received a approval recommendation from the European Medicines Agency. The patent protection expired in 2016.
In April 2013, the Supreme Court of India, the highest Indian court, ruled in a landmark ruling that imatinib mesilate, the active ingredient of Glivec , is not granted patent protection in India. In 2006, the Indian authorities had already denied Novartis the patenting of imatinib mesilate. The Indian Patent Act of 2005 excludes patent protection for medicinal substances in Paragraph 3 (d) if they represent only a slight modification (e.g. salts, isomers or polymorphic crystal structures ) of already existing medicinal substance molecules and this modification does not result in any efficacy advantage leads. Novartis countered this by stating that the modification compared to the already known imatinib base was not minor, but serious.
The Indian Paragraph 3 (d) regulation is actually intended to prevent the extension of existing patent protection with only minimal changes to the previously patented active ingredient ( evergreening ). However, Imatinib-Base was not protected by patents, since patents on medicinal substances were generally not granted in India before 2005. For the finished drug Glivec , Novartis was granted exclusive marketing rights for a period of five years in 2003 based on a special clause of the then applicable Indian patent law, the Indian Patent Act 1970 .
Further areas of application
In addition to the treatment of various types of cancer, imatinib mesilate was also intended for the additional treatment of pulmonary arterial hypertension (PAH). However, Novartis withdrew an application for approval in this area of application after the data required to assess drug safety could not be submitted within the time period specified by the authorities.
Trade names
Imatinib is commercially available in the EU countries and Switzerland under the name Glivec .
Web links
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Imatinib
literature
- M. Deininger et al. a .: The development of imatinib as a therapeutic agent for chronic myeloid leukemia. In: Blood . Volume 105, 2005, pp. 2640-2653, PMID 15618470 .
- SG O'Brien et al. a .: Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. In: N. Engl. J. Med. Volume 348, 2003, pp. 994-1004, PMID 12637609 .
- DG Savage, KH Antman: Imatinib Mesylate - A New Oral Targeted Therapy. In: N. Engl. J. Med. Volume 346, 2002, pp. 683-693, PMID 11870247 .
Individual evidence
- ↑ a b c d E. B. Atici, B. Karliga: Quantitative determination of two polymorphic forms of imatinib mesylate in a drug substance and tablet formulation by X-ray powder diffraction, differential scanning calorimetry and attenuated total reflectance Fourier transform infrared spectroscopy. In: J. Pharm. Biomed. Anal. 114 (2015) 330-340, doi: 10.1016 / j.jpba.2015.06.011 .
- ↑ a b Registration dossier on benzamides, 4 - [(4-methyl-1-piperazinyl) methyl] -N- [4-methyl-3 - [[4- (3-pyridinyl) -2-pyrimidinyl] amino] phenyl] - ( GHS section ) from the European Chemicals Agency (ECHA), accessed on July 3, 2020.
- ↑ Oliver Hantschel, Uwe Rix, Giulio Superti-Furga: Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib . In: Leukemia & Lymphoma . tape 49 , no. 4 , January 1, 2008, p. 615-619 , doi : 10.1080 / 10428190801896103 , PMID 18398720 .
- ↑ Patent WO 99 / 03854A (Novartis, January 28, 1998).
- ↑ Patent EP2604596 : Polymorphs of imatinib. Published on June 19, 2013 , Inventors: Philipp Daniel Haas, Fikret Koc, Bekir Karliga, Atici Esen Bellur, Ramazan Sivasligil.
- ↑ Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Imatinib Teva .
- ↑ Bitter pill for Novartis Ärzte Zeitung online, October 23, 2012.
- ↑ Public Assessment Report (EPAR) of the European Medicines Agency (EMA) on: Imatinib Actavis .
- ↑ apotheke adhoc: Glivec will be generic ( memento of the original from March 4, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , February 27, 2013.
- ↑ a b Novartis is ultimately subject to In: Neue Zürcher Zeitung. from April 1, 2013.
- ↑ Novartis' imatinib patent battle resumes in Indian Supreme Court. In: thepharmaletter.com. from August 15, 2011.
- ^ Ducking 'TRIPS in India: A Saga Involving Novartis and the Legality of Section 3 (d). In: National Law School of India Review. Volume 20, Number 2, 2008, pp. 131-155.
- ↑ Pharmaceutical industry in the country background. In: Tages-Anzeiger of August 22, 2012.
- ↑ J. Bidder: Protection of intellectual property, Focus online, January 25, 2008.
- ↑ Declaration from Bern: Novartis files two lawsuits in India: Cancer patients and health groups demand the withdrawal of the lawsuits ( Memento of July 14, 2010 in the Internet Archive ) (PDF; 25 kB), September 26, 2006.
- ↑ EMA press release: Novartis Europharm Ltd withdraws its marketing-authorization application for Ruvise (imatinib mesilate) , January 24, 2013.
