Limes
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
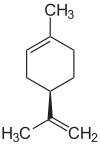
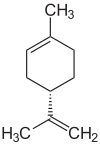
| ( R ) - (+) - Limonene (left) and ( S ) - (-) - Limonene | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Limes | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 16 | |||||||||||||||
| Brief description |
colorless, flammable liquid, characteristic lemon odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 136.24 g · mol -1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.84 g cm −3 (α and β form, 20 ° C) |
|||||||||||||||
| Melting point |
−89 ° C |
|||||||||||||||
| boiling point |
175 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
little in water (14 mg l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
D -limons:
|
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Limonene [ limoˈneːn ] is a natural product from the terpene group (monocyclic monoterpene).
structure
Limonene occurs in the form of two enantiomers , the ( R ) - (+) - limonene (also known as D - (+) - limonene or (+) - limonene for short) and the ( S ) - (-) - limonene [also referred to as L - (-) - limonene or (-) - limonene for short]. The racemate of the two enantiomers is also called dipentene .
| Limes | ||||
| Surname | D- limes | L- limes | ||
| other names | (+) - Lime ( R ) -Lime |
(-) - Lime ( S ) -Limons |
||
| Structural formula |

|

|
||
| CAS number | 5989-27-5 | 5989-54-8 | ||
| EC number | 227-813-5 | 227-815-6 | ||
| ECHA info card | 100.025.284 | 100.025.286 | ||
| PubChem | 440917 | 439250 | ||
| Wikidata | Q27888324 | Q27089405 | ||
history
Limonene was first made in 1878 by Gustave Bouchardat by heating isoprene .
Occurrence
Limonene is the most common monoterpene found in plants . ( R ) - (+) - limonene is especially bitter orange , in cumin oil , in dill oil in coriander oil in lemon oil (about 65%) and Orange oil contained (usually> 90%). It has an orange-like odor. In contrast, ( S ) - (-) - limonene is found in silver fir and in peppermint oil and smells like turpentine . The racemic limonene occurs, among other things, in pine oil , Siberian spruce needle oil , neroli oil , nutmeg oil and camphor oil .
Extraction / representation
Limonene is obtained primarily through natural substance extraction. ( R ) - (+) - Limonene occurs in large quantities as a by-product in orange juice production and is obtained by steam distillation of the peel. ( S ) - (-) - Limonene is extracted from the corresponding oils in relatively small amounts. The racemic limonene is obtained as a by-product in the acid-catalyzed isomerization of α- and β- pinene .
biosynthesis
The biosynthesis of limonene starts from geranyl pyrophosphate (GPP).
properties
Physical Properties
The specific angle of rotation is [α] 20 D + 126.3 ° [ D -limons] or −126.3 ° [( S ) -limons].
Chemical properties
Limonene is sensitive to light, air, heat, alkalis and acids and auto-oxidizes to carvone .
Two successive reactions with oxygen and carbon dioxide produce polylimone carbonate, a substance with properties similar to polystyrene . It is the starting material for the synthesis of β- seline . In the first step it reacts with diborane and is then oxidized with hydrogen peroxide .
Safety-related parameters
Limonene forms flammable vapor-air mixtures at higher temperatures. The compound has a flash point of 50 ° C. The explosion range is between 0.7% by volume (39 g / m 3 ) as the lower explosion limit (LEL) and 6.1% by volume (345 g / m 3 ) as the upper explosion limit (UEL). The limit gap width was determined to be 1.14 mm. This results in an assignment to explosion group IIA. The ignition temperature is 255 ° C. The substance therefore falls into temperature class T3.
use

Limonene is traditionally used as an inexpensive fragrance . Today it is mainly used as a biogenic solvent and serves as a cleaner and thinner, for example in the paint industry .
The gram-negative bacterium Pseudomonas putida DSM 12264 is able to oxidize D - (+) - limonene regioselectively to D - (+) - perillaic acid , a natural preservative for cosmetics . The biotechnological production of D - (+) - perillic acid from D - (+) - limonene on a laboratory scale was improved in 2010. The developed bioprocess represents a promising option for industrial application.
The D - (+) - limonene is used as a herbal insecticide .
It is also used as a starting material for the synthesis of synthetic THC ( dronabinol ). In more recent processes, limonene is also used as a raw material for bioplastics .
Biological importance
When metabolism of limonene mainly arises Perillinsäure , Dihydroperillinsäure , limonene-1,2-diol and Uroterpenol . Lime has an irritant effect. Its oxidation products D - (-) - carvone and several isomers of limonene oxide , which arise from limonene in the air, are allergenic.
safety instructions
D - (+) - limonene has been classified as non- carcinogenic to humans .
Web links
- Entry on Limonene in the Spectral Database for Organic Compounds (SDBS) of the National Institute of Advanced Industrial Science and Technology (AIST)
Individual evidence
- ↑ a b c d e f g h i j k l m n o p Entry on Dipenten in the GESTIS substance database of the IFA , accessed on February 18, 2017(JavaScript required) .
- ^ A b c R. T. O'Connor, LA Goldblatt: Correlation of Ultraviolet and Infrared Spectra of Terpene Hydrocarbons , in: Analytical Chemistry . 26, 1954, pp. 1726-1737; doi: 10.1021 / ac60095a014 .
- ↑ Entry on (±) -1-methyl-4- (1-methylvinyl) cyclohexene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 5989-27-5 or D-limonene ), accessed on November 2, 2015.
- ↑ Entry on (D) - (+) - limonene in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on July 31, 2018 or earlier.
- ↑ Entry on lemon oil. In: Römpp Online . Georg Thieme Verlag, accessed on June 15, 2014.
- ↑ Entry on limes. In: Römpp Online . Georg Thieme Verlag, accessed on June 15, 2014.
- ↑ Rosaria Ciriminna, Monica Lomeli-Rodriguez, Piera Demma Carà, Jose A. Lopez-Sanchez and Mario Pagliaro: Limonene: a versatile chemical of the bioeconomy , Chem. Comm. , 2014, 50, pp. 15288-15296, doi: 10.1039 / c4cc06147k .
- ↑ a b c d e E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ Juliane Daphi-Weber, Heike Raddatz, Rainer Müller: Investigation of Fragrances - Controlled Fragrances , pp. 94–95, in Volume V of the series HighChem hautnah - News from food chemistry (published by the Society of German Chemists ) 2010, ISBN 978- 3-936028-64-5 .
-
^
Ruben Eckermann: With bacteria against bacteria . In: News from chemistry . tape 59 , 2011, p. 619-620 .
For the biotechnological production of perillic acid, the working group of industrial research associations "Otto von Guericke" (AiF) awarded the Otto von Guericke Prize 2011 to Jens Schrader from the Karl Winnacker Institute (today DECHEMA Research Institute ).
- ↑ MA Mirata et al .: Integrated bioproduction and selective purification of perillic acid , chemistry engineer technology . 82, 2010, pp. 101-109.
- ↑ ApSimon: "The Total Synthesis of Natural Products" Vol. 4 John Wiley & Sons, New York Chichester Brisbane Toronto, p. 233.
- ↑ Bähr, M .; Bitto, A .; Mühlhaupt, R .: Cyclic limonene dicarbonate as a new monomer for non-isocyanate oligo- and polyurethanes (NIPU) based upon terpenes . In: Green Chemistry 14 (1012), pp. 1447-1454, doi: 10.1039 / C2GC35099H .
- ↑ Firdaus, M .; Meier, MAR: Renewable polyamides and polyurethanes derived from limonene . In: Green Chemistry 15 (1013), pp. 370-380, doi: 10.1039 / C2GC36557J .
- ^ AT Karlberg et al. (1992): Air oxidation of d-limonene (the citrus solvent) creates potent allergens. In: Contact Dermatitis . Vol. 26, pp. 332-340, PMID 1395597 .
- ^ Entry on limes in the Hazardous Substances Data Bank , accessed on March 3, 2010.





