Methacrylic anhydride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Methacrylic anhydride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 10 O 3 | |||||||||||||||
| Brief description |
clear colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 154.16 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
−109 ° C |
|||||||||||||||
| boiling point | ||||||||||||||||
| Vapor pressure |
90 Pa (20 ° C ) |
|||||||||||||||
| solubility |
soluble in water (98 g l −1 at 20 ° C ),> 10% in ethanol and ether in chloroform , benzene , toluene , tetrahydrofuran |
|||||||||||||||
| Refractive index |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Methacrylic acid anhydride or methacryloyl anhydride is a bifunctional α, β-unsaturated carbonyl compound and, as an anhydride of methacrylic acid and functional alkene, is a very reactive acylating agent and monomer .
Methacryloyl anhydride reacts with water to form methacrylic acid and in contact with polymerization initiators it polymerizes to polymethacrylic anhydride.
Manufacturing
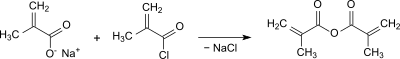
Methacryloyl chloride reacts in the classic way with sodium methacrylate to form methacrylic anhydride.
Methacryloyl anhydride can also be obtained by reacting sodium methacrylate with benzoyl chloride in a modest 43% yield.
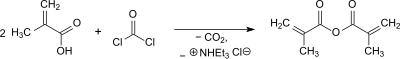
Methacrylic anhydride is formed in the reaction of methacrylic acid with phosgene in the presence of triethylamine as an acid scavenger and hydroquinone as a polymerization inhibitor with a yield of 90.3%.
Because of the relatively expensive and unpleasant (methacryloyl chloride is highly irritating to tears) or toxic (phosgene) reactants, the processes described are not suitable for the production of methacrylic anhydride on an industrial scale.
The dehydration of methacrylic acid by reaction with acetic anhydride is extensively described, particularly in the patent literature, in batch reactions and in reactions carried out continuously. The reaction takes place in two equilibrium reactions, first to give the mixed anhydride of methacrylic and acetic acid (1), which reacts with other acetic anhydride to form methacryloyl anhydride (2).
The gross reaction (3) describes the " re- anhydridization" taking place with acetic anhydride . To achieve a reasonable space-time yield, the acetic acid formed must be removed from the equilibrium as quickly and gently as possible by distillation.
The tendency of methacrylic acid and methacrylic anhydride to polymerize (especially at elevated temperatures and longer reaction times, even in the presence of polymerisation inhibitors!) And to form heavy (higher molecular weight) by-products through Michael addition , as well as to thermal decomposition of the products formed, reduces the selectivity of the reaction and increases the risk of deposits forming in the reactor ( fouling ). The reaction is therefore carried out at reduced pressure, e.g. B. at 100 mmHg, partly in the presence of a catalyst, such as. B. chromium (III) acetate or other metal acetates, sufficient amounts of polymerization inhibitors, such as. B. phenothiazine and hydroquinone and the gradual addition of acetic anhydride at boiling temperatures between 80 and 95 ° C (depending on the internal pressure of the reaction) carried out until practically complete conversion of the acetic anhydride. The different process variants deliver raw yields of approx. 60 to 80% of methacrylic anhydride.
The distillative separation of the multicomponent mixture of acetic acid (boiling point 118 ° C), acetic anhydride (139.4 ° C), methacrylic acid (161 ° C), methacrylic anhydride (197 ° C) and the mixed anhydride (209.9 ° C) under reduced pressure is usually carried out after the solid by-products have been separated off and delivers pure (approx. 95%) to highly pure (> 99.9%) product.
With reactive distillation , which is relatively demanding in practice , 99.95% of the methacrylic acid can be converted from the reaction mixture, if necessary with the addition of an entrainer , under optimized conditions and a methacrylic anhydride yield of 86.99% can be achieved.
The synthesis described in a patent application from methyl methacrylate and carbon monoxide in the presence of rhodium (III) chloride , triphenylphosphine and methyl iodide gives up to 18.2% by weight methacrylic anhydride at 100 bar and 200 ° C. and is therefore technically irrelevant.
properties
In its pure state, methacrylic anhydride is a clear, colorless liquid, irritating to breath, irritating to the eyes, and corrosive with a pungent odor. The substance is soluble in water, but hydrolyzes rapidly in water in an exothermic reaction to methacrylic acid. Because of its tendency to polymerize, methacrylic anhydride is treated with effective amounts of an inhibitor, such as. B. 2,4-dimethyl-6-tert-butylphenol stabilized.
Applications
Reactions with low molecular weight compounds
Despite its lower reactivity towards nucleophiles compared to acid chlorides, methacrylic anhydride is a useful (since it is milder) reagent for the formation of esters, thioesters or amides with the introduction of the polymerization-active methacryloyl group. However, as with all acid anhydrides, half of the anhydride molecule is lost as methacrylic acid for the acylation reaction.
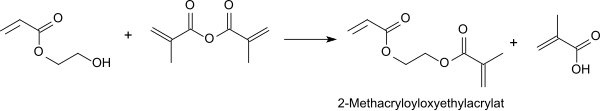
Methacrylic anhydride reacts with 2-hydroxyethyl acrylate in the presence of a strongly acidic ion exchanger at room temperature smoothly in yields of> 90% to form 2-methacroyloxyethyl acrylate, which z. B. can be used as an asymmetric (meth) acrylate crosslinker in superabsorbents .
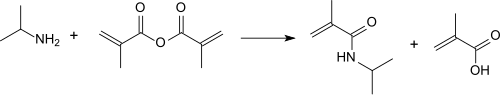
Methacrylic anhydride reacts with isopropylamine at temperatures below 30 ° C in high yields (> 90%) and purities (> 97%) to form N -isopropyl methacrylamide.
Longer-chain amines, such as. B. dodecylamine, can be converted to the homologous methacrylamides with methacryloyl anhydride according to the same procedure in excellent yields (99%) and purities (98%).
Homo- and copolymerization reactions
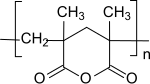
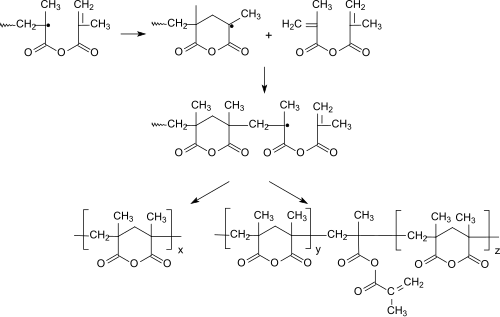
Methacrylic anhydride polymerizes in the presence of radical initiators to form polymethacrylic anhydride, the polymer chain of which is mainly composed of six-membered rings.
More detailed investigations showed that the cyclopolymerization at high temperatures, low monomer concentrations, "bad", ie. H. little solvating, solvents and preferentially takes place at high conversion and gives readily soluble cyclic homopolymers. The "normal" vinyl polymerization of methacrylic anhydride, which occurs as a competitive reaction, requires the incorporation of polymerization-active methacryloyl groups, which lead to crosslinking and lower solubility of the cyclopolymer.
By suitable combination of a lithium ester enolate with a spatially demanding aluminum bisphenoxide, methacrylic anhydride can also be anionically cyclopolymerized at −78 ° C to form a largely syndiotactic macromolecule.
Also in copolymers, e.g. B. with vinyl monomers such as styrene , methyl methacrylate or methyl acrylate , methacrylic anhydride forms six-membered cyclic structures.
Individual evidence
- ↑ a b c d e Entry on methacrylic anhydride in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c Data sheet methacrylic acid anhydride from Sigma-Aldrich , accessed on May 19, 2017 ( PDF ).
- ↑ a b c d Datasheet Methacrylic anhydride at AlfaAesar, accessed on December 15, 2014 ( PDF )(JavaScript required) .
- ↑ a b c D.R. Lide: Handbook of Chemistry and Physics, 76th ed., 1995-1996 . CRC Press, Boca Raton, 2010, ISBN 978-1-4398-2077-3 , pp. 3-291 .
- ↑ a b c Evonik Industries AG, GPS Safety Summary, Methacrylic anhydride (MAAH) , July 2013, Online = [1]
- ↑ X. Xiong, S.-X. Qu, Y.-M. Liu: Synthesis and characterization of dopamine graft compound N-methacrylyoyl-3,4-dihydroxyl-phenylamine . In: J. Phys .: Conf. Ser. tape 419 , 2013, p. 012047 , doi : 10.1088 / 1742-6596 / 419/1/012047 .
- ^ A b T. Brotherton, J. Smith, Jr., J. Lynn: Notes-Acrylic and Methacrylic Anhydrides . In: J. Org. Chem. Band 26 , no. 4 , 1961, pp. 1283-1284 , doi : 10.1021 / jo01063a072 .
- ↑ JCH Hwa, WA Fleming, L. Miller: Acrylic anhydrides and polymers derived therefrom . In: J. Polym. Sci., A Gen. Pap. Tape 2 , no. 5 , 1964, pp. 2383-2400 , doi : 10.1002 / pol . 1964.100020527 .
- ↑ Patent WO9532940 : Process for the production of olefin acid anhydrides and esters thereof. Applied May 30, 1995 , published December 7, 1995 , Applicant: Howard University, Inventor: F. Ayorinde, M. Hassan.
- ↑ Patent US7074957 : Process for preparing (meth) acrylic anhydride. Applied on June 28, 2002 , published on July 11, 2006 , applicant: Arkema, inventor: B. Dupont, J.-M. Paul.
- ↑ Patent EP0196520 : Process for the production of carboxylic acid anhydrides. Filed on March 14, 1986 , published on October 8, 1986 , Applicant: BASF AG, inventor: K. Bott, A. Anderlohr, T. Faust, J. Guth.
- ↑ Patent US8097757 : Method for preparing (meth) acrylic anhydride. Applied on January 27, 2009 , published on January 17, 2012 , applicant: Arkema France, inventor: J.-M. Paul, S. Tretjak.
- ↑ Patent EP1231201A1 : Process for the production of anhydrides of unsaturated carboxylic acids. Registered on January 29, 2002 , published on August 14, 2002 , applicant: Röhm GmbH & Co. KG, inventors: B. Schmitt, J. Knebel, W. Klesse, A. Wittkowski, B. Laux.
- ↑ Patent US8586791B2 : Method for producing (meth) acrylic anhydride, method for storing (meth) acrylic anhydride, and method for producing (meth) acrylate. Applied on August 4, 2009 , published on November 19, 2013 , applicant: Mitsubishi Rayon Co., Ltd., inventor: R. Ansai, H. Nogami, K. Ogura.
- ↑ H. Wang, X. Bu, Z. Huang, J. Yang, T. Qiu: Synthesis of methacrylic anhydride by batch reactive distillation: reaction kinetics and process . In: Ind. Eng. Chem. Res. Volume 53 , no. 44 , 2014, p. 17317-17324 , doi : 10.1021 / ie501607v .
- ↑ Patent EP0226942A2 : Process for the production of unsaturated aliphatic carboxylic acid anhydrides. Registered on December 9, 1986 , published on July 1, 1987 , applicant: Röhm GmbH, inventor: K. Langerbein.
- ↑ Patent US6881858B2 : Asymmetric (meth) acrylate crosslinking agents. Registered on January 11, 2002 , published on April 19, 2005 , applicant: Roehm GmbH & Co. KG, inventor: W. Siol.
- ↑ Patent US20110218312A1 : Method for producing N-isopropyl (meth) acrylamides. Registered on November 19, 2009 , published on September 8, 2011 , applicant: Evonik Roehm GmbH, inventors: J. Knebel, W. Karnbrock, V. Kerscher.
- ↑ G. Smets, P. Hous, N. Deval: Cyclopolymerization. IV. Structure of polymethacrylic anhydride and kinetics of polymerization of methacrylic anhydride . In: J. Polym. Sci., A, Gen. Pap. Tape 2 , no. 1 , 1964, pp. 4825-4834 , doi : 10.1002 / pol . 1964.100021113 .
- ^ TF Gray, Jr., GB Butler: The fundamental basis for cyclopolymerization. X. A systematic study of the cyclopolymerization of methacrylic anhydride . In: J. Macromol. Sci. A., Chemistry . tape 9 , no. 1 , 1975, p. 45-82 , doi : 10.1080 / 00222337508068646 .
- ↑ T. Kitaura, N. Moroi, T. Kitayama: Anionic cyclopolymerization of methacrylic anhydride with the aid of bulky aluminum Lewis acid . In: polymer . tape 54 , no. 8 , 2013, p. 1987-1992 , doi : 10.1016 / j.polymer.2012.12.078 .
- ↑ JCH Hwa, L. Miller: Copolymerization of methacrylic anhydride with vinyl monomers . In: J. Polym. Sci. tape 55 , no. 161 , 1961, pp. 197-213 , doi : 10.1002 / pol . 1961.1205516120 .
- ↑ FC Baines, JC Bevington: Studies of the polymerization and copolymerization of methacrylic anhydride . In: polymer . tape 11 , no. 12 , 1970, pp. 647-658 , doi : 10.1016 / 0032-3861 (70) 90017-0 .
- ↑ GB Butler: Cyclopolymerization and Cyclocopolymerization . Marcel Dekker, Inc., 1992, ISBN 0-8247-8625-4 , pp. 251 ff .