Myxobolus cerebralis
| Myxobolus cerebralis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

Myxobolus cerebralis |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Myxobolus cerebralis | ||||||||||||
| Hofer , 1903 |
Myxobolus cerebralis is a parasite from the Myxozoa group . It attacks trout fish (Salmonidae) such as trout , char and salmon and causes rotation sickness in them. The earliest descriptions of the disease come from stocks of rainbow trout in Germany around 1900, the first description of the parasite by Bruno Hofer was in 1903. Since then the disease has spread worldwide and is now in almost all of Europe including Russia , the USA and South Africa spread. In the 1980s it became known that the parasite needed Tubifex tubifex , an annelid from the group of Tubificidae, as an intermediate hostfor its development. It attacks its ultimate host, the fish, by throwing a pile thread out of a capsule thatresemblesa nettle capsule, whichpenetrates the host's skin. Myxobolus cerebralis was the first species of Myxozoa to be scientifically described.
morphology
M. cerebralis occurs in various morphological stages that range from individual cells to relatively complex spores. Some of the stages have not yet been fully explored.
Triactinomyxon stage
The triactinomyxon stage is the stage at which the fish become parasitized. It consists of a long shell about 150 micrometers long and three long appendages or "tails", each 200 micrometers long. At the end of the long bowl there is a sporoplasm package with 64 germ cells that are surrounded by other cells. There are also three polar capsules, each containing a wound pile filament with a length of 170 to 180 micrometers. These filaments can be shot in this as well as in the Myxospora stage and form an opening in the tissue of the fish, into which the sporoplasm penetrates.
Sporoplasm stage
After contact with the fish and the shooting of the pile filaments, the sporoplasm, which consists of amoeboid cells, penetrates the tissue. Here it divides mitotically and in this way produces further amoeboid cells, which penetrate further into the tissue and into the nervous tissue .
Myxospora stage
Lenticular myxospora with a diameter of about 10 micrometers, each consisting of six cells, develop in the cell tissue within the host. Two of these cells form polar capsules, two more are changing in a two polynuclear sporoplasm and the two last again form an envelope about as Valve is called. These Myxospora penetrate into the intermediate hosts, the Tubificiden, which feed on the remains of the dead fish.
Life cycle
Myxobolus cerebralis is a generational parasite that needs two different hosts for its development: a trout fish and a tubificid. The only known worm in which Myxobolus cerebralis can develop is Tubifex tubifex , although this may not represent a single species, but a complex of species.
The worms ingest the myxospores as they feed on the tissue of the dead, infected fish. In the intestines of the worms, the myxospores anchor themselves through the pile filament in the inner lining. Here the valves open and the binuclear cell tissue leaves the shells and penetrates between the epithelial cells of the intestine. The germ cells multiply and produce more amoeboid cells through an asexual mechanism known as merogony . Through this process, a single ingested parasite can infect the cell spaces of 10 consecutive segments of the worm. It is believed that complete cells can be formed from cell parts here.
After 60 to 90 days, the parasite forms sexual stages that form spores in the form of pansporocytes, each containing eight Triactinomyxon stages. These leave the worm through its anus into the open water and can infect a fish through the skin here. Affected tubificids can excrete adult Triactinomycetes in this way for over a year. Alternatively, fish that eat tubificids with parasites can also be infected. In this case, the infection happens through the intestinal wall. This process only takes a few seconds, during which the fish is first penetrated by the pile filaments and infected with the sporoplasm. Within a few hours, the asexual cell division of the sporoplasm begins into further amoeboid cells that spread in the fish tissue.
In fish, the parasites reproduce through an asexual endogony , in which new cells are created in old parasite cells. The final stage in fish is the Myxospora stage. This is only released again when the fish has died and decomposed or when it is eaten. According to more recent findings, however, it is perhaps also possible that myxospores are released while the fish is still alive. The myxospores are very resistant to environmental influences. Experiments have shown that the spores survive even if they are frozen for three months at −20 degrees Celsius. In the mud they remain infectious for about 5 months and they also survive an intestinal passage in ducks undamaged. The Triactinomyxone, however, live a maximum of 34 days, depending on the temperature.
pathology
Rotational sickness occurs in juvenile fish and causes deformation of the skeleton and damage to the central nervous system . As a result, it is no longer possible for the fish to swim normally through the water, instead they move forward in a spiral. They are easier prey for predators and can only hunt with great difficulty. About 90 percent of the infected fish die as "finger cots" and the surviving animals remain deformed in both the skeleton and the tissue. The parasites continue to live in the fish until they naturally die after they are released back into the open water. Due to the high mortality rate , Myxobolus cerebralis is one of the most dangerous pathogens and at the same time the largest economic pests of the fishing industry . A transfer to humans is not possible.
So far, infection with Myxobolus cerebralis has been detected in a number of species of trout fish. The infection is certain in eight species of the genus Salmo , four species of the genus Oncorhynchus , four species of char ( Salvelinus ) as well as the European grayling ( Thymallus thymallus ) and the huchen ( Hucho hucho ). Damage to the fish occurs through the penetration of the parasites and their spread in the tissue as well as the fact that the parasites feed on the fish tissue.
Outwardly recognizable, the caudal fin becomes darker and the skeleton is deformed due to the destruction of the tissue on the bones that are being formed. In addition, the described swimming disorders occur, which are eponymous for the rotational sickness and are caused by damage to the spinal cord and the brain stem . The internal organs are normally undamaged, but tissue damage can be seen in the muscle tissue. Experiments have shown that the immune system of the fish combats and can kill invading spores, but that there is no longer an immune reaction as soon as the parasites have established themselves in the nervous system. The immune reaction depends on the species.
The parasite infestation is not fatal for the Tubifex tubifex worm . This mainly results in damage to the intestinal mucous membranes due to the release of the parasites from the intestinal wall. Since this happens several thousand times in a worm, it also affects food intake. Infected worms are usually smaller and less colored than uninfected representatives. The parasites only leave the worm at water temperatures between 10 and 15 ° C, so that fish in colder or warmer waters cannot be infected. The infestation rate fluctuates accordingly with the seasons.
susceptibility
The susceptibility of the fish to parasite infestation depends on their age, size and of course the concentration of the parasite spores in the water. The water temperature also plays a major role. The most vulnerable are fish less than five months old, in which the skeleton is not yet fully ossified. This also makes the animals more prone to deformation. The species belonging to the fish also plays an important role. Studies have shown that rainbow trout and brook trout are parasitized much more frequently than other types of trout, while king salmon , brown trout and arctic grayling were hardly affected.
The brown trout also shows hardly any symptoms when infected with the parasite, which is why it is assumed that this is the original host. The parasite's widespread distribution therefore only occurred when the brown trout came into contact with newly introduced species such as the rainbow trout ( neozoa ), which are more susceptible to the disease.
diagnosis
A strong to very strong infection with the parasites can be recognized externally by the deformations mentioned and the change in behavior. This usually happens 35 to 80 days after infection, but an insufficient supply of tryptophan or ascorbic acid can have similar effects. A clear diagnosis is therefore only possible if myxospores are detected in the tissue of the fish.
In the case of severe infestation, this can easily be demonstrated by examining the tissue with a microscope . If the infestation is lower, a sample of the tissue is digested with the proteases pepsin and trypsin in order to make the spores recognizable. The tissue is then examined for typical signs of myxobolus cerebralis infection. A serological examination of the tissue with the help of specific antibodies is also possible. Another reliable method is to search for specific genes in tissue, using a polymerase chain reaction with special markers to search for a known gene on the 18S rRNA .
These examinations are routinely carried out in different regions, especially where the parasite can cause more damage. In Australia and Canada , where parasites have not yet appeared, the tests are used to identify and combat an infestation at an early stage.
Spread

While Myxobolus cerebralis was only a harmless fish parasite of brown trout in Europe and a few other species in Asia for a long time, it has also spread due to the strong global expansion of rainbow trout. As the number of parasitized rainbow trout increased, so did the number of spores formed, causing the waters to become more contaminated. With the much higher number of parasites, the infection pressure on the less susceptible species also increased; these too could now be decimated considerably by the parasite. In some areas this has led to a sharp decline in fish populations or even to the complete disappearance of individual species.
Spread in Europe
As already mentioned, Europe represents the natural range of the parasite and native species are adapted to parasitization by Myxobolus cerebralis . In these species, the disease is usually very mild and without any noticeable symptoms. The parasite was only able to spread dramatically through the very vulnerable rainbow trout. There are only very few wild populations of these fish in European waters; rather, the stocks are regularly supplemented by sport fishermen , as the trout are popular fishing animals. In response to the severe parasitization, the breeding conditions in Europe were adapted accordingly. The young fish are raised in water that is certainly spore-free and they are only released when the ossification of the skeleton is completely complete and the animals are no longer susceptible to the parasites.
Spread in New Zealand
The first parasites were discovered in New Zealand in 1971, although the findings were limited to rivers in the South Island and were therefore not close to the economically important fish stocks and farms. The domestic trout fish were also not susceptible to the parasite, so that it did not spread to any great extent. However, the discovery led to severe restrictions on the export of fish to the neighboring state of Australia, where importation of the parasite should be prevented.
Spread in the USA
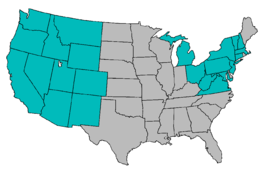
In North America, Myxobolus cerebralis was first discovered in Pennsylvania in 1956 . The parasite was introduced through fish imports from Europe and has since spread mainly south and west. Until the 1990s, turning sickness was only a problem in the rainbow trout fish farms, which at times could easily be kept under control. Since then, however, the parasite has fully established itself in the natural waters of some areas and is a serious problem especially in the areas of the Rocky Mountain states ( Colorado , Wyoming , Utah , Montana , Idaho , New Mexico ). In some rivers of these Areas, the stock of trout and salmon declined by up to 90 percent. The areas where sport fishing makes up a large proportion of tourism income are particularly affected by the turning sickness. For example, the damage in Montana is estimated at around 300 million US dollars. In addition, some of the trout species affected are now threatened with extinction and have completely disappeared in some regions.
Combat
In order to get the parasite epidemic under control, some biologists have started looking for ways that the spores can be effectively controlled. The main aim is to find a way to get the polar capsules to prematurely shoot the pile threads so that they can no longer be used against fish. In laboratory tests it was found that the spores only reacted with a launch at high concentrations of acids or bases , the addition of salt or electricity. Neither neurochemicals , substances that sensitize the capsules in cnidarians, nor the slime of trout, narcotics or dead fish triggered the capsules. Even if substances are found that cause a release, the question arises whether they can also be used outdoors.
Another approach uses the different susceptibility of fish, which is sometimes very pronounced within the species. With the help of particularly resistant breeding lines , the susceptibility of the fish in the waters should be reduced.
In addition, fish farming is no longer using any potentially contaminated sediments and thus keeping the fish farming basins free from parasites, as has already been done successfully in Europe. Regular disinfection of the substrate is intended to reduce or completely prevent the proliferation of Tubificiden . Completely substrate-free tanks, in which worms cannot keep, are also in use.
Drug treatment of fish is also an option, but it cannot be used in wild fish populations. You can choose from furazolidone , furoxone , benomyl , fumagillin , proguanil and clamoxyquin . In experiments, the addition of fumagillin was able to reduce the parasite infestation from originally 73 to 90 percent to 10 to 20 percent in rainbow trout.
Taxonomy
The scientific name cerebralis comes from the early idea that the parasite described primarily affects the central nervous system and the brain (cerebrum) of the host. After it was determined that this is not the case and the parasite is instead to be found in the tissue and there especially on the skeleton, it should be renamed Myxobolus chondrophagus . However, this is not possible due to the zoological nomenclature rules . In addition, it was found that organisms that were previously listed as Triactinomyxon dubium and T. gyrosalmo in a separate class Actinosporea are stages of the Myxobolus cerebralis (Triactinomyxon stage), which accordingly also received this name.
literature
- Andree, KB, MacConnell, E. and Hedrick, RP (1998): A nested polymerase chain reaction for the detection of genomic DNA of Myxobolus cerebralis in rainbow trout Oncorhynchus mykiss , Diseases of Aquatic Organisms 34, 145-54
- Bartholomew, JL and Reno, PW (2002): The history and dissemination of whirling disease , American Fisheries Society Symposium 29; 3-24
- Bergersen, EP, and Anderson, DE (1997): The distribution and spread of Myxobolus cerebralis in the United States , Fisheries 22 (8); 6-7
- El-Matbouli, M., and Hoffmann, RW (1991): Effects of freezing, aging, and passage through the alimentary canal of predatory animals on the viability of Myxobolus cerebralis spores , Journal of Aquatic Animal Health 3; 260-262
- El-Matbouli, M., and Hoffmann, RW (1998): Light and electron microscopic studies on the chronological development of Myxobolus cerebralis to the Actinosporean stage in Tubifex Tubifes, International Journal For Parasitology 28; 195-217
- El-Matbouli, M., Hoffman, RW, Shoel, H., McDowell, TS, & Hedrick, RP (1999): Whirling disease: host specificity and interaction between the actinosporean stage of Myxobolus cerebralis and rainbow trout ( Oncorhynchus mykiss ) cartilage , Diseases of Aquatic Organisms 35; 1-12
- Gilbert, MA & Granath, WO Jr. (2003): Whirling disease and salmonid fish: life cycle, biology, and disease , Journal of Parasitology 89; 658-667
- Halliday, MM (1976): The Biology Of Myxosoma cerebralis : The Causative Organism Of Whirling Disease Of Salmonids , Journal of Fish Biology 9; 339-357
- Hedrick, RP & El-Matbouli, M. (2002): Recent advances with taxonomy, life cycle, and development of Myxobolus cerebralis in the fish and oligochaete hosts , American Fisheries Society Symposium 29; 45-53
- Hoffmann, G. (1962): Whirling Disease Of Trout , US Department Of The Interior, Fishery Leaflet 508; 1-3
- Lom, J. & Dyková, I. (1992): Protozoan Parasites of Fishes , Elsevier , Amsterdam ( ISBN 0444894349 )
- Markiw, ME (1989): Portals of entry for salmonid whirling disease in rainbow trout , Diseases of Aquatic Organisms 6; 6-10
- Markiw, ME (1992): Salmonid Whirling didease , Fish and Wildlife Leaflet 17, full text ( Memento from July 10, 2004 in the Internet Archive )
- Markiw, ME (1992): Experimentally induced whirling disease. II. Determination of longevity of the infective triactinomyxon stage of Myxobolus cerebralis by vital staining , Journal of Aquatic Animal Health 4; 44-47
- Nehring, RB (1996): Whirling Disease In Feral Trout Populations In Colorado , in EP Bergersen And BAKnoph (Ed.): Proceedings: Whirling Disease Workshop –– where Do We Go From Here? , Colorado Cooperative Fish And Wildlife Research Unit, Fort Collins
- Nehring, RB Thompson, KG Taurman, KA & Shuler, DL (2002): Laboratory studies indicating that living brown trout Salmo trutta expel viable Myxobolus cerebralis myxospores , American Fisheries Society Symposium 29; 125-134
- Nickum, D. (1999): Whirling Disease in the United States: A Summary of Progress in Research and Management , Trout Unlimited.
- Tennyson, J. Anacker, T. & Higgins, S. (1997): Scientific breakthrough helps combat trout disease. US Fish and Wildlife Service Whirling Disease Foundation News Release.
- Vincent, ER (1996): Whirling Disease — the Montana Experience, Madison River , in, EP Bergersen And BAKnoph (Ed.): Proceedings: Whirling Disease Workshop — where Do We Go From Here? , Colorado Cooperative Fish And Wildlife Research Unit, Fort Collins
- Vincent, ER (2002): Relative susceptibility of various salmonids to whirling disease with emphasis on rainbow and cutthroat trout , American Fisheries Society Symposium 29; 109-115
- Wagner, EJ Cannon, Q. Smith, M. Hillyard, R. & Arndt, R. (2002): Extrusion of Polar Filaments of the Myxobolus cerebralis Triactinomyxon by salts, electricity, and other agents , American Fisheries Society Symposium 29; 61-76
- Wisconsin Department of Agriculture, Trade and Consumer Protection, Division of Animal Health (October 2001): Fish Health Advisory: Whirling Disease in Trout. ( PDF )
Web links
- Report of the World Trade Organization (WTO) on the import restrictions of trout to Australia (English, DOC format; 1.0 MB)
- The Whirling Disease Foundation (English)