Cooling
As cooling processes in which an object or a system of objects are called, heat is withdrawn. The objects can be solid or semi-solid (e.g. loose rock ), but also fluids (liquids, gases) or a combination thereof.
Theory (thermodynamics)
In the case of solids and liquids, the heat transfer (or the extraction of heat) takes place according to the temperature gradient . Three processes work to different degrees:
- Heat conduction (mainly for solids and liquids),
- Thermal radiation (in nature mainly solar radiation and nocturnal radiation),
- and (mostly weaker) the ad or convection .
The result of these processes is a temperature equalization (see 3rd sentence of thermodynamics ). It always results in an increase in the total entropy - i. H. a conversion of various forms of energy into thermal energy.
Continuous (cooling) cooling is therefore impossible in a closed system: The thermal energy of an externally insulated system can only increase, but not decrease. In the technical field z. B. a refrigerator can only cool its interior , while the average temperature of the entire room increases.
Further influencing variables in heating or cooling processes are:
- the convection (in solids zero for gases usually high, for liquids in the middle area)
- the coefficient of thermal conductivity and the heat capacity
- the adiabatic cooling , for example in the Earth's atmosphere at discounts and thermals
- the mean temperature gradient of the atmosphere (average 5–7 ° C per kilometer) and the humidity
- generally the gas laws and the Joule-Thomson effect
- mean weather and precipitation
- the albedo (reflection coefficient) of the soil and plants (also dependent on precipitation)
- occasionally processes of condensation and resublimation .
Cooling processes in nature
In natural systems, the most effective forms of cooling are
- the nightly cooling by radiation ,
- the advection (contact with objects colder)
- cooling through evaporation (removal of the heat of evaporation )
- and ventilation (wind, movement).
The speed at which the temperature of the body or object drops depends on
- the external conditions (effective temperature difference, humidity , radiation conditions, wind, friction, etc.)
- as well as the thermal properties of the body or fluid ( specific heat , surface properties, porosity , etc.),
- and its possible isolation (soil, weathered or tree bark, fur, plumage).
Cool off at night
At night the air temperature drops i. a. to some degree, in extreme climates and up to 30 ° C . The reason for this is the radiation (infrared or thermal radiation ) of the heated floor , which is particularly effective when the sky is clear. The soil, which is warmed up during the day by solar radiation and tends to achieve thermal equilibrium in the early afternoon, generally cools down more slowly than the air, but faster than water . This leads to thermal inversions or the formation of local wind systems that are almost opposite to the daytime winds (see land-sea wind system near the sea coast).
The nocturnal cooling abruptly decreases or even reverses when clouds gather in the night sky or the dew point is reached. In the first case, the radiation is reduced because the clouds have an isolating effect; in the second case, the condensation heat of the mist droplets is supplied to the air.
The difference between day and night temperature is usually recorded by a temperature curve or by specifying maximum and minimum temperatures. The temperature maximum occurs in Central Europe at around 2 p.m. CET (or 3 p.m. summer time), the temperature minimum usually just before sunrise (see also morning dew ).
While the temperature profile near the ground has the amplitude mentioned of a few degrees to tens of degrees, the ground temperature is already noticeably more balanced at a depth of a few centimeters. At a depth of 1 meter (see also frost depth ) or in caves, the daily, but also the seasonal temperature change can largely disappear, which depends on the soil properties and geology, vegetation , water content and climatic conditions.
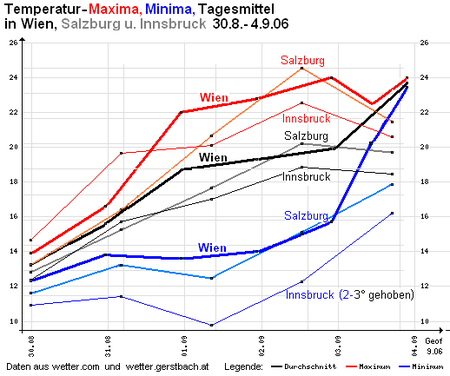
In special weather conditions, the difference between day and night temperatures can be almost zero, and when a warm front passes through it can even be reversed. The illustration on the right shows an example of an unusual weather situation with exceptionally little nighttime cooling.
Cooling by technical measures
In technology, the planned cooling is referred to as cooling . You can z. B.
- serve to establish a state of thermal equilibrium ,
- for dissipating frictional or lost heat from moving or otherwise heated components ,
- and in particular to avoid overheating.
- Cooling can be determined by temperature measurement , equalized by thermostats or heating or its effect can be modeled by calibration . Preservation purposes for biomaterial.
- The highest cooling rates are achieved with melt spinning ( )
See also
- Heat flow , water , air cooling
- Heat sink (technical and biological), cooling size
- Freezing capacity , solidification , pourability
- Astronomical refraction , vertical gradient
- Heat pump , chiller
- Hypothermia