Phentermine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Phentermine | ||||||||||||||||||
| other names |
2-methyl-1-phenyl-propan-2-amine |
||||||||||||||||||
| Molecular formula | C 10 H 15 N | ||||||||||||||||||
| Brief description |
|
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 149.23 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
202–205 ° C (hydrochloride) |
||||||||||||||||||
| boiling point |
100 ° C (28 h Pa ) |
||||||||||||||||||
| pK s value |
10.23 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Phentermine is a phenylalkylamine that is used as an appetite suppressant and psychostimulant .
Presentation and extraction
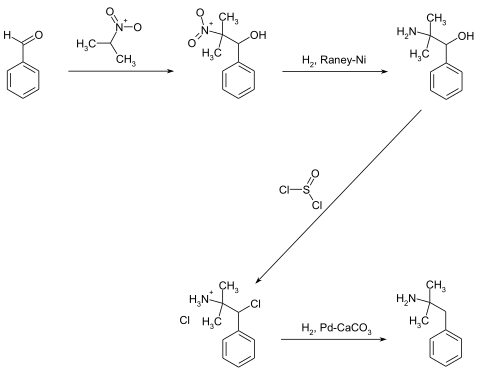
Phentermine is made in four steps. The synthesis starts from benzaldehyde , to which 2-nitropropane is added in the first step in the sense of a Henry reaction . The resulting 2-methyl-2-nitro-1-phenyl-1-propanol is then hydrogenated to the amine over Raney nickel . In the third step, thionyl chloride is used to carry out nucleophilic substitution of the OH group to form 2-amino-2-methyl-1-phenylpropyl chloride. The target compound is obtained in the last step by a palladium- catalyzed hydrogenation.
pharmacology
When phentermine is an amphetamine - derivative , its mechanism of action on the influence of neurotransmitters is based in the human brain. It stimulates bundles of neurons to release catecholamines - including dopamine , adrenaline (epinephrine), and norepinephrine . The mechanism of action corresponds to that of other appetite suppressants, such as diethylpropion and phendimetrazine . The hunger signal is partially suppressed, but this effect weakens after a few weeks with continued use. Phentermine, like amphetamines, can cause psychological dependence and is addictive.
Admission
Phentermine has not been approved in Germany since the early 1970s. The active ingredient is part of the appetite suppressant Fen-Phen, a combination of phentermine and fenfluramine , which was mainly marketed in the USA at the time , which is believed to be responsible for the fact that some test persons developed heart valve changes . As a result of these incidents, the fenfluramine that was blamed for it was completely withdrawn from the US market. There are now thousands of product liability lawsuits pending in the United States regarding this drug .
Phentermine has been approved in connection with the active ingredient topiramate on the American market under the Qsymia brand as an appetite suppressant for the treatment of obesity since 2012 . The European Medicines Agency considers the risk of the preparation even after a re-evaluation is still higher than one the potential benefits, which is why approval was not made.
Trivia
Bubba Smith , known from the Police Academy film series, died on August 3, 2011 of a phentermine overdose. The actor had taken the medicine despite existing heart problems and high blood pressure.
Individual evidence
- ↑ a b c d Entry on phentermine. In: Römpp Online . Georg Thieme Verlag, accessed on October 26, 2014.
- ↑ External identifiers or database links for phentermine hydrochloride : CAS number: 1197-21-3, EC number: 214-821-9, ECHA InfoCard: 100.013.474 , PubChem : 70969 , ChemSpider : 64130 , Wikidata : Q19885635 .
- ↑ I. Canals, K. Valkó, E. Bosch, AP Hill, M. Rosés: retention of Ionizable compounds on HPLC. 8. Influence of Mobile-Phase pH Change on the Chromatographic Retention of Acids and Bases during Gradient Elution , in; Anal. Chem. 2001 , 73 , 4937-4945; doi: 10.1021 / ac0101454 .
- ↑ a b Datasheet Phentermine hydrochloride from Sigma-Aldrich , accessed on April 19, 2011 ( PDF ).
- ↑ Entry on phentermine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ A b c d e A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications , 4th edition (2000), Thieme-Verlag Stuttgart, ISBN 978-1-58890- 031-9 .
- ↑ Patent US2408345 (Marrell 1946).
- ↑ Data analysis prompted withdrawal of fenfluramine / dexfenfluramine . In: Inpharma Weekly . tape 1107 , no. 1 , October 1, 1997, p. 21-21 , doi : 10.2165 / 00128413-199711070-00045 .
- ^ FDA: Press Announcements> FDA approves weight-management drug Qsymia .
- ↑ Refusal of the marketing authorization for Qsiva (phentermine / topiramate) (PDF; 107 kB).
- ↑ blick.ch: "Police Academy" star: "Hightower" died of an overdose of diet pills - TV - Blick .