Thioflavine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Thioflavine T | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Thioflavine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 17 H 19 ClN 2 S | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 318.86 g mol −1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Thioflavin T is a benzothiazole- based cationic dye that is used as a potent marker for amyloidosis in histology . It is also used to study the electrical fluctuation in bacterial biofilms .
Production of thioflavin T
Thioflavin T is the trimethyl chloride of dehydrothiotoluidine [6-methyl-2- (4-aminophenyl) benzothiazole]. The dehydrothiotoluidine is accessible by reacting p -toluidine with sulfur at an elevated temperature. This is converted to thioflavin T by methylation with methanol in the presence of hydrochloric or sulfuric acid.
Use Thioflavin T
Thioflavin T can be used as a dye for dyeing silk and tannin cotton .
The use of thioflavin in histology was first described in 1959 by pathologists Philip S.Vassar and Charles F. Culling of the Department of Pathology at the University of Vancouver. Thioflavin T was used for the histological examination of kidney tumors for amyloidosis. In a subsequent report in 1961, they described two thyroid carcinomas obtained by hemithyroidectomy . In both samples, the treatment with thioflavin T produced a bright yellow-green fluorescent coloration in the stoma of the tumors, which can be clearly seen under a fluorescent microscope using ultraviolet light .
Further research showed that thioflavin T interacts with a large number of tissue components. These each generate specific coloring patterns. Tissue types include mucopolysacarids, nucleic acids, and many structures contained in amyloid fibrils. These properties are used particularly in the diagnosis of Alzheimer's disease . The study of the fluorescent staining of senile but not diffuse plaques in the affected regions of Alzheimer's syndrome, as well as the green-birefringent Congo-red staining of the same structures were established as anatomical neuropathological criteria for the disease. The diagnosis of amyloid fibrils can be made in vivo , ex vivo, or in vitro .
The structure of Thioflavin T has a hydrophobic end with a dimethylamino group which is in para position to a polar benzothiazole group on a phenyl group . Through this combination of polar and hydrophobic regions, the thioflavin T molecules form micelles with hydrophobic interiors in aqueous solutions . The positively charged nitrogen atom points to the solvent. As a result, hydrogen bonds form between the thioflavin T micelles and the tissue structures.
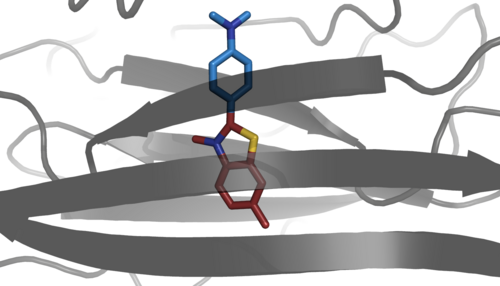
The binding of thioflavin T to the amylidose fibrils causes a red shift of the excitation spectrum from 336 nm of the free dye in aqueous solution to an excitation maximum of 450 nm of the bound dye. This characteristic hyperchromic shift in fluorescence excitation occurs only in amyloidoses, which have the multimeric fibrillar forms of the β-sheet polymers. Only the β-sheet polymers offer a suitable environment for stabilizing the electronic ground state of the dye, which results in the large red shift of the excitation maximum. Another study names the electrostatic interaction between double-stranded DNA and thioflavin T as a possible cause of a redshift in the same order of magnitude, which, however, depends on the concentration of the DNA and can be eliminated by the presence of sodium chloride .
The change in the spectrum of the dye results from rotational immobilization of the phenyl group. In free solution, it behaves like a molecular rotor. In the free molecule, a non-fluorescent TICT process (twisted intramolecular charge transfer ) competes with a fluorescent LE state (locally excited). According to this, the TICT process changes the torsion angle between the phenyl group and the benzothiazole group in the excited singlet state from 37 ° to 90 °. This process effectively competes with the radiation transition of the LE state and thereby extinguishes the fluorescence. However, when the thioflavin T molecule is bound to an amyloid, this rotation is stopped, thereby suppressing the quenching of the fluorescent LE state.
Thioflavin T produces an exceptionally specific or characteristic fluorescence in amyloidosis, the only false-positive result of thioflavin T in this spectrum occurred with polysaccharides in the aortic wall in Marfan's syndrome .
Genetic studies with roundworms of the species Caenorhabditis elegans have shown that the use of thioflavin T can slow down the aggregation of protein fibrils. This can lead to a significantly longer service life and a slower aging process. Thioflavin T also suppresses pathological features of mutated metastable proteins and β-amyloid-associated toxicity in humans. This can lead to pharmaceutical maintenance of the protein homeostasis network, which can have a profound effect on aging and on age-related diseases.
Thioflavin S
Thioflavin S is obtained by sulfation during the methylation of the purine base with sulfuric acid. It is a complex mixture of compounds whose structures have not been characterized.
Since the binding of thiofalvin S to the amyloid fibrils increases the emission intensity many times over, but without changing the excitation and emission spectra, this leads to a high background fluorescence. This makes this dye unsuitable for quantitative analysis in solution.
The way thioflavone S binds to amyloidosis is probably the same as that of Congo red .
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A labeling of 2- [4- (dimethylamino) phenyl] -3,6-dimethylbenzothiazolium chloride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on January 5, 2019, is reproduced from a self-classification by the distributor .
- ↑ Biosynth Direct Store: Thioflavin T - CAS: 2390-54-7 - Basic Yellow 1 - All Products - Biosynth Direct Store , accessed January 15, 2019.
- ↑ Molecular mechanism of Thioflavin-T binding to amyloid fibrils . In: Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics . tape 1804 , no. 7 , July 1, 2010, ISSN 1570-9639 , p. 1405–1412 , doi : 10.1016 / j.bbapap.2010.04.001 , PMID 20399286 , PMC 2880406 (free full text).
- ↑ Gürol M. Süel, Jordi Garcia-Ojalvo, San Ly, Munehiro Asally, Jintao Liu: Ion channels enable electrical communication in bacterial communities . In: Nature . tape 527 , no. 7576 , November 2015, ISSN 1476-4687 , p. 59–63 , doi : 10.1038 / nature15709 , PMID 26503040 , PMC 4890463 (free full text).
- ^ Rudolf Nietzki : Chemistry of organic dyes. 5th edition, Julius Springer, Berlin 1906, p. 289 ( digitized version ).
- ↑ Basic Yellow 1. http://www.worlddyevariety.com , accessed January 2, 2019 .
- ^ A b c Klaus Hunger (Ed.): Industrial Dyes: Chemistry, Properties, Applications . WILEY-VCH Verlag, Weinheim 2003, ISBN 978-3-662-01950-4 , p. 261 ( limited preview in Google Book search).
- ↑ Matthew Biancalana, Shohei Koide: Molecular mechanism of Thioflavin-T binding to amyloid fibrils . In: Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics . tape 1804 , no. 7 , July 2010, ISSN 1570-9639 , p. 1405-1412 , doi : 10.1016 / j.bbapap.2010.04.001 .
- ^ Philip S. Vassar, Charles F. Culling: The Significance of Amyloid in Carcinoma of the Thyroid Gland . In: American Journal of Clinical Pathology . tape 36 , no. 3 , September 1, 1961, ISSN 0002-9173 , p. 244-247 , doi : 10.1093 / ajcp / 36.3.244 .
- ↑ a b Harry LeVine: Thioflavine T interaction with amyloid β-sheet structures . In: amyloid . tape 2 , no. 1 , January 1995, ISSN 1350-6129 , pp. 1-6 , doi : 10.3109 / 13506129509031881 .
- ↑ a b Mechanism of thioflavin T binding to amyloid fibrils . In: Journal of Structural Biology . tape 151 , no. 3 , September 1, 2005, ISSN 1047-8477 , p. 229-238 , doi : 10.1016 / j.jsb.2005.06.006 .
- ↑ hyperchromia. In: Lexicon of Physics. Spektrum.de logo, accessed on February 28, 2019 .
- ↑ emission of thioflavin T and its control in the presence of DNA . In: Journal of Photochemistry and Photobiology A: Chemistry . tape 162 , no. 1 , February 20, 2004, ISSN 1010-6030 , p. 129-137 , doi : 10.1016 / S1010-6030 (03) 00320-4 .
- ↑ Vitali I. Stsiapura, Alexander A. Maskevich, Valery A. Kuzmitsky, Konstantin K. Turoverov, Irina M. Kuznetsova: Computational Study of Thioflavine T Torsional Relaxation in the Excited State . In: Journal of Physical Chemistry A . tape 111 , no. June 22 , 2007, ISSN 1089-5639 , pp. 4829-4835 , doi : 10.1021 / jp070590o .
- ↑ ES Voropai, MP Samtsov, KN Kaplevskii, AA Maskevich, VI Stepuro: Spectral Properties of Thioflavin T and Its Complexes with Amyloid Fibrils . In: Journal of Applied Spectroscopy . tape 70 , no. 6 , November 2003, ISSN 0021-9037 , p. 868-874 , doi : 10.1023 / b: japs.0000016303.37573.7e .
- ↑ SM Saeed, Gerald Fine: Thioflavin-T for Amyloid Detection . In: American Journal of Clinical Pathology . tape 47 , no. 5 , May 1, 1967, ISSN 0002-9173 , pp. 588-593 , doi : 10.1093 / ajcp / 47.5.588 .
- ↑ GABRIEL KELIÉNYI: ON THE HISTOCHEMISTRY OF AZO GROUP-FREE THIAZOLE DYES . In: Journal of Histochemistry & Cytochemistry . tape 15 , no. 3 , March 1967, ISSN 0022-1554 , pp. 172-180 , doi : 10.1177 / 15.3.172 .
- ↑ a b Harry LeVine: [18] Quantification of β-sheet amyloid fibril structures with thioflavin T . In: Methods in Enzymology . Elsevier, 1999, ISBN 978-0-12-182210-1 , pp. 274-284 , doi : 10.1016 / s0076-6879 (99) 09020-5 .
- ↑ Harry Levine: Thioflavine T interaction with synthetic Alzheimer's disease β-amyloid peptides: Detection of amyloid aggregation in solution . In: Protein Science . tape 2 , no. 3 , December 31, 2008, ISSN 0961-8368 , p. 404-410 , doi : 10.1002 / pro.5560020312 .


