Thionyl fluoride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
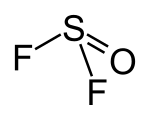
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Thionyl fluoride | ||||||||||||||||||
| other names |
Thionyl difluoride |
||||||||||||||||||
| Molecular formula | F 2 OS | ||||||||||||||||||
| Brief description |
colorless gas |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 86.06 g mol −1 | ||||||||||||||||||
| Physical state |
gaseous |
||||||||||||||||||
| density |
1.780 g cm −3 (−100 ° C) |
||||||||||||||||||
| Melting point |
−110.5 ° C |
||||||||||||||||||
| boiling point |
−43.7 ° C |
||||||||||||||||||
| solubility |
slow hydrolysis in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Thionyl fluoride is a chemical compound from the group of thionyl halides .
Extraction and presentation
Thionyl fluoride can be obtained by reacting thionyl chloride with hydrogen fluoride or sodium fluoride .
The simplest representation in the laboratory is the reaction of thionyl chloride with antimony (III) fluoride in the presence of antimony (V) chloride as a catalyst :
properties
Thionyl fluoride is a colorless gas that is thermally resistant to red heat. Base metals such as magnesium , nickel , mercury and aluminum are not attacked in a dry atmosphere of up to 125 ° C. While glass is attacked from 400 ° C, no reaction occurs with iron at this temperature. It is only slowly hydrolyzed by ice-cold water and smokes lightly on contact with moist air. In the solid state it has a monoclinic crystal structure with the space group P 2 1 / c (space group no. 14) . It reacts with fluorine to form thionyl tetrafluoride F 4 SO. It is readily soluble in diethyl ether and benzene .
Individual evidence
- ↑ a b c d e f g Georg Brauer (Ed.), With the collaboration of Marianne Baudler u. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 187.
- ↑ Entry on thionyl fluoride in the GESTIS substance database of the IFA , accessed on July 10, 2016(JavaScript required) .
- ↑ a b c d W. C. Smith and EL Muetterties: Thionyl fluoride . In: Eugene G. Rochow (Ed.): Inorganic Syntheses . tape 6 . McGraw-Hill Book Company, Inc., 1960, pp. 162-163 (English).
- ^ Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer DE, 1998, ISBN 3-642-58842-5 , pp. 712 ( limited preview in Google Book search).
- ↑ Catherine E. Housecroft: Inorganic Chemistry . Pearson Education, 2005, ISBN 0-13-039913-2 , pp. 450 ( limited preview in Google Book Search).




![{\ displaystyle {\ ce {3 SOCl2 + 2 SbF3 -> [SbCl_5] [] 3 SOF2 + 2 SbCl3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a1c2d5ebe96edb8f2850ce7dac7420ae90bf7eb1)
