Triptolide
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
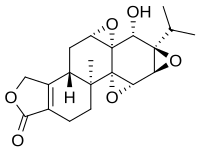
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Triptolide | |||||||||||||||||||||
| other names |
PG490 |
|||||||||||||||||||||
| Molecular formula | C 20 H 24 O 6 | |||||||||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 360.40 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
226-227 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Triptolide , also known as PG490 , is a diterpene with three epoxide groups contained in the Chinese climbing plant Wilford's trifoliate fruit ( Tripterygium wilfordii ) .
Occurrence
Triptolide is the most important bioactive component of the more than 300 different ingredients of the Chinese spindle tree family Tripterygium wilfordii .
synthesis
Although several - including stereoselective - routes to the total synthesis of triptolide are described in the literature , the easiest way to obtain it is as a natural product by extraction from the spindle tree family Tripterygium wilfordii . The plant components are extracted with ethanol and the various active ingredients are separated by subsequent chromatographic processes.
Discovery and Isolation
Triptolide was first isolated and characterized in 1972 from the extract of Tripterygium wilfordii by a research group led by S. Morris Kupchan at the University of Virginia .
Pharmacological properties
Triptolide is a potent immunosuppressant and anti-inflammatory agent.
In tumor cells , but also in T lymphocytes , it can initiate programmed cell death ( apoptosis ) by activating the DEVD- cleaving caspase 3 ; but not with thymocytes. Triptolide also sensitizes the TNF-α -induced apoptosis in tumor cells and inhibits the TNF-α-induced activation of the nuclear factor κB . Triptolide also inhibits the MAP kinase pathway ( mitogen- activated protein - kinase ) MAPK1. Triptolide inhibits the expression of interleukin-2 in T lymphocytes .
Triptolide also has an antileukemic effect in the mouse and dog animal models .
Long -term administration of triptolide, or an extract from Tripterygium wilfordii, can cause reversible sterility in men, as sperm count and mobility are reduced. This effect was first observed when several Chinese were treated with T. wilfordii extract. The men were treated for rheumatoid arthritis and psoriasis with a daily dose of 20 to 30 mg of the extract and became sterile after about two months. The testosterone - and LH -mirror were just as libido and potency unchanged. One to two months after treatment discontinuation, the men returned to normal fertility. One of the reasons for this effect of triptolide is that it inhibits the calcium channels of the spermatogenic cells. One possible application that has been discussed and tested in initial clinical studies is the hormone-free pill for men . In the rat animal model , however, the infertility was no longer reversible, which is why interest in triptolide for this application has waned.
Triptolide is a potential drug for the treatment of patients with cystic kidneys . In various model organisms - similar to the immunosuppressant sirolimus (rapamycin) - promising results were obtained.
Compared to a number of cancer cell lines ( in vitro ) as well as in the model organism ( in vivo ) triptolide shows a high cytostatic effectiveness. So it is able to reduce the growth of tumors and metastases . Triptolide is effective in concentrations of 2 to 10 ng / ml ( in vitro ) and is more effective than, for example, the chemotherapeutic agent Paclitaxel (Taxol). As a result of these findings, various clinical studies with triptolide as a chemotherapeutic agent were started. Due to the high toxicity, triptolide and its related ingredients of T. wilfordii have only a small therapeutic range . Various derivatives have been made to reduce toxicity . With the help of Aspergillus niger , structural modifications were obtained which show a high degree of effectiveness against human cancer cell lines in vitro . A water-soluble triptolide derivative called PG490-88 is currently in Phase I clinical for the treatment of solid tumors.
Triptolide binds in the organism to PC2, a 110 kDa heavy protein .
Individual evidence
- ↑ Triptolide data sheet (PDF) from Calbiochem, accessed on December 8, 2015.
- ↑ WZ Gu u. a .: Isolation, purification, and characterization of immunosuppressive compounds from tripterygium: triptolideand tripdiolide. In: Int J Immunopharma. 17/1995, pp. 351-356. PMID 7591358
- ↑ Data sheet Triptolide from Tripterygium wilfordii from Sigma-Aldrich , accessed on May 9, 2017 ( PDF ).
- ↑ Entry on triptolide in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ a b c D. Biermann: Looking for active ingredients in China's herb chambers. In: Pharmaceutical newspaper . 32/2008.
- ↑ AM Brinker et al .: Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). In: Phytochemistry . 68/2007, pp. 732-766. PMID 17250858
- ^ Kwan-wah Pang: Studies Toward Stereoselective Total Synthesis of Triptolide. University of Hong Kong, 1997.
- ↑ JP Demers: The Total Synthesis of Triptolide. Stanford University, 1980.
- ↑ J. ApSimon et al: The Total Synthesis of Natural Products. Wiley-Interscience, ISBN 0-471-54507-4 , pp. 82-89.
- ↑ E. Hackenthal Triptolide. In: Hager's Handbook of Pharmaceutical Practice. Springer, 1999, ISBN 3-540-62646-8 .
- ↑ SM Kupchan et al .: Triptolide and triptolidine, novel antileukemia diterpenoid triepoxides from Tripterygium wilfordii. In: JACS 94/1972, pp. 7194-7195, PMID 5072337 .
- ↑ MA Chan et al .: Triptolide is more effective in preventing T cell proliferation and interferon-gamma production than is FK506. In: Phytotherapy Research . 13/1999, pp. 464-467, PMID 10479754 .
- ↑ H. Lu et al .: Immunosuppressive effect of triptolide in vitro. In: Transplant. Proc. 31/1999, pp. 2056-2057, PMID 10455969 .
- ↑ a b D Qiu et al: Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box / nuclear factor of activated T-cells and NF-kappaB transcriptional activation. In: Journal of Biological Chemistry 274/1999, pp. 13443-13450, PMID 10224109 .
- ↑ D. Qiu, PN Kao: Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. In: Drugs RD 4/2003, pp. 1-18, PMID 12568630 .
- ↑ Y. Yang et al .: Triptolide induces apoptotic death of T lymphocyte. In: Immunopharmacology 40/1998, pp. 139-149, PMID 9826028 .
- ↑ KY Lee et al .: PG490 (Triptolide) Cooperates with Tumor Necrosis Factor- to Induce Apoptosis in Tumor Cells. In: J. Biol. Chem. 274/1999, pp. 13451-13455, PMID 10224110 .
- ↑ Q. Zhao et al: The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. In: J. Biol. Chem. 280/2005, pp. 8101-8108, PMID 15590669 .
- ^ EG Shepherd et al.: The function of mitogen-activated protein kinase phosphatase-1 in peptidoglycan-stimulated macrophages. In: J. Biol. Chem. 279/2004, pp. 540234-54031, PMID 15485842 .
- ↑ S. Jäger et al.: Pharmacology of selected terpenes. In: Pharmazeutische Zeitung 22/2006.
- ↑ BJ Chen: Triptolide, a novel immunosuppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii hook F. In: Leukemia & Lymphoma 42/2001, pp. 253-265.
- ^ Y. Lue et al.: Triptolide: a potential male contraceptive. In: J. Androl. 19/1998, pp. 479-486, PMID 9733151 .
- ↑ S. Czajka: Biosyntheses to tinker with. In: Pharmaceutical Newspaper 1999.
- ^ CE Hoesl et al .: Reversible, non-barrier male contraception: status and prospects. In: Eur. Urol. 48/2005, pp. 712-722, PMID 16230226 .
- ^ SA Matlin et al.: Male antifertility compounds from Tripterygium wilfordii Hook f. In: Contraception 47/1993, pp. 387-400. PMID 8508668
- ^ AW Meikle: Endocrine Replacement Therapy in Clinical Practice. Humana Press, 2003, ISBN 1-58829-195-2 , p. 437.
- ^ Triptolide: A Potential Drug For Polycystic Kidney Disease. In: Science Daily of March 12, 2007.
- ↑ Experiments Point To New Treatments For PKD. In: Science Daily, April 6, 2008.
- ↑ SJ Leuenroth, N. Bencivenga, P. Igarashi, S. Somlo, CM Crews: Triptolide reduces cystogenesis in a model of ADPKD. In: J. Am. Soc. Nephrol. 19, 2008, pp. 1659-1662; PMID 18650476 ; PMC 251844 (free full text).
- ↑ a b S. J. Leuenroth et al .: Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. In: Proc Natl Acad Sci 104/2007, pp. 4389-4394, PMID 17360534 ; PMC 1838612 (free full text).
- ↑ S. Yang et al: Triptolide inhibits the growth and metastasis of solid tumors. In: Mol. Cancer Ther. 2/2003, pp. 65-72, PMID 12533674 .
- ↑ TM Kiviharju et al .: Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. In: Clin Cancer Res 8/2002, pp. 2666-2674, PMID 12171899 .
- ↑ L. Ning et al. a: Cytotoxic biotransformed products from triptonide by Aspergillus niger. In: Planta Med 96/2003, pp. 804-808. PMID 14598204 .
- ↑ R. Loch: Antimicrobial Agents as Potential Antitumor Agents. (PDF; 1.5 MB) Dissertation, Albert-Ludwigs-University Freiburg i. Br., 2007.
- ↑ JM Fidler et al: PG490-88, a derivative of triptolide, causes tumor regression and sensitizes tumors to chemotherapy. In: Mol. Cancer Ther. 2/2003, pp. 855-862, PMID 14555704 .
- ↑ SJ Leuenroth, CM Crews: Studies on calcium dependence reveal multiple modes of action for triptolide. In: Chem Biol 12/2005, pp. 1259-1268, PMID 16356843 ; PMC 2486259 (free full text).
literature
- SM Kupchan, RM Schubert: Selective alkylation: a biomimetic reaction of the antileukemic triptolides? In: Science 185/1974, pp. 791-793, PMID 4843378 .
- BJ Chen: Triptolide, a novel immunosuppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. In: Leuk Lymphoma 42/2001, pp. 253-265, PMID 11699390 .
- WB Schill among others: Men's medicine. Elsevier GmbH Germany, 2004, ISBN 3-437-23260-6 .
- F. DiCosmo et al: Plant Cell Culture Secondary Metabolism: Toward Industrial Application. CRC Press, 1996, ISBN 0-8493-5135-9 .
- YS Wei, I. Adachi: Inhibitory effect of triptolide on colony formation of breast and stomach cancer cell lines. In: Chung Kuo Yao Li Hsueh Pao 12/1991, pp. 406-410. PMID 1819894 .
- LA Shamon et al: Evaluation of the mutagenic, cytotoxic, and antitumor potential of triptolide, a highly oxygenated diterpene isolated from Tripterygium wilfordii. In: Cancer Lett 112/1997, pp. 113-117, PMID 9029176 .
- You-Ping Zhu: Chinese Materia Medica: Chemistry, Pharmacology and Applications. Harwood Academic, 1998, ISBN 90-5702-285-0 .
- EW Smith, HI Maibach: Percutaneous Penetration Enhancers. CRC Press, 2005, ISBN 0-8493-2152-2 .
- M. Rai, MC Carpinella: Naturally Occurring Bioactive Compounds. Elsevier, 2006, ISBN 0-444-52241-7 .
further reading
- WZ Gu, SR Brandwein: Inhibition of type II collagen-induced arthritis in rats by triptolide. In: Int J Immunopharmacol 20/1998, pp. 389-400.
- N. Lin et al .: Triptolide, a novel diterpenoid triepoxide from Tripterygium wilfordii Hook. f, suppresses the production and gene expression of pro-matrix metalloproteinases 1 and 3 and augments those of tissue inhibitors of metalloproteinases 1 and 2 in human synovial fibroblasts. In: Arthritis Rheum 44/2001, pp. 2193-2200.
- B. Wang et al .: Triptolide, an active component of the Chinese herbal remedy Tripterygium wilfordii Hook F, inhibits production of nitric oxide by decreasing inducible nitric oxide synthase gene transcription. In: Arthritis Rheum 50/2004, pp. 2995-3003.