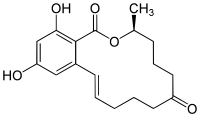
Zearalenone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Zearalenone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 18 H 22 O 5 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 318.36 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
187-189 ° C |
||||||||||||||||||
| solubility | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Zearalenone (abbreviated to ZEA or ZON) is a widely used mycotoxin . It belongs to the group of substances called Fusarium toxins . Chemically, it can be understood as a macrolide and resorcylic acid derivative ( resorcinol -α- carboxylic acid derivative), so it is a macrocyclic lactone . The biosynthesis takes place via a polyketide path.
Occurrence
Zearalenon is formed by different species of the ubiquitous genus Fusarium . Relevant is the occurrence of the toxin in crops that have been attacked by Fusarium graminearum or Fusarium culmorum - especially maize , wheat , barley and the like. a. Grain . Zearalenone is always accompanied by mycotoxins from the group of trichothecenes such as deoxynivalenol in the affected plants .
properties
Zearalenone is a white, crystalline substance. It is so thermally and chemically stable that its content in contaminated food is not significantly reduced either by storage or preparation (cooking, baking).
use
A large number of derivatives of zearalenone have been synthesized and tested for their pharmacological effectiveness. The 6- (6,10-dihydroxy-undecyl) -β-resorcylic acid lactone, known as α-zearalanol, which results from zearalenone by reducing the double bond and the keto group, is used as a growth promoter for cattle. This use has been banned in the EU since 1989 .
Biological importance
Zearalenone acts as an estrogen . Its binding affinity for estrogen receptors is about ten to twenty times less than that of 17-β- estradiol , but its half-life is significantly longer. A constant intake of food leads to hyperestrogenism with all its symptoms and consequences: increase in size and weight of the uterus , disruption of the menstrual cycle , pathological changes in the ovaries , pseudo-pregnancies , abortions and sterility . These effects occur particularly in pigs and humans , while cattle are less affected and hardly any effects are observed in chickens .
There is also reason to believe that zearalenone is the cause of premature pubertal changes in children. Due to its estrogenic effect, it has an influence on the tumor formation of hormonally sensitive tissue (see e.g. breast cancer ).
The estrogen effect of zearalenone is further increased by its metabolism: a reductive restructuring takes place in the body, which leads to four different derivatives (including the above-mentioned α-zearalanol), which are predominantly much more effective by reducing the keto group and the double bond .
safety instructions
Zearalenone has only a low acute toxicity ( LD 50 (mouse, oral )> 2000 mg / kg, LD 50 (rat, oral)> 10000 mg / kg). These values cannot, in fact, be achieved through the consumption of contaminated food. Due to the estrogen effect, a TDI ( tolerable daily intake ) of 0.2 µg / kg was decided by the Scientific Committee on Food of the European Commission . The statutory maximum levels for zearalenone are 50 µg / kg or 100 µg / kg (for corn-based products) for cereal products and 20 µg / kg especially for cereal products for the production of dietetic foods for infants and young children.
See also
further reading
- C. Böhm: Development of an immuno-analytical method for the sample preparation of Deoxynivalenol and Zearalenone in food and feed. (PDF; 658 kB) Dissertation, University of Vienna, 2009
Web links
- The Scientific Committee on Food of the European Commission on zearalenone (PDF file; 78 kB)
- Report for the European Food Safety Authority ( Memento from August 16, 2005 in the Internet Archive )
Individual evidence
- ↑ a b Entry on zearalenone. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ a b c d Zearalenone data sheet from Sigma-Aldrich , accessed on April 25, 2011 ( PDF ).
- ↑ Regulation (EG) No. 1881/2006 Appendix Section 2.5 (consolidated version of March 19, 2018)


