Tafluprost: Difference between revisions
→References: Added content Tags: canned edit summary Mobile edit Mobile web edit |
Filled in 0 bare reference(s) with reFill 2 |
||
| (26 intermediate revisions by 17 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical compound}} |
|||
{{Use dmy dates|date=April 2024}} |
|||
{{Drugbox |
{{Drugbox |
||
| IUPAC_name = |
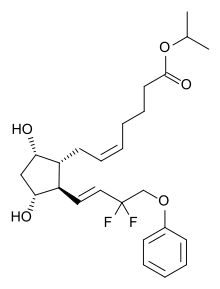
| IUPAC_name = Isopropyl (5''Z'')-7-<nowiki/>{(1''R'',2''R'',3''R'',5''S'')-2-[(1''E'')-3,3-difluoro-4-phenoxybut-1-en-1-yl]-3,5-dihydroxycyclopentyl}hept-5-enoate |
||
| image = Tafluprost_structure.svg |
| image = Tafluprost_structure.svg |
||
<!--Clinical data--> |
<!--Clinical data--> |
||
| tradename = Saflutan, Taflotan |
| tradename = Saflutan, Taflotan, Zioptan |
||
| Drugs.com = {{drugs.com| |
| Drugs.com = {{drugs.com|MTM|tafluprost-ophthalmic}} |
||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
||
| pregnancy_US = C |
|||
| pregnancy_category = |
| pregnancy_category = |
||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> |
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> |
||
| legal_CA = |
| legal_CA = Rx-only |
||
| legal_CA_comment =<ref>{{cite web |title=Product monograph |url=https://pdf.hres.ca/dpd_pm/00058012.PDF |website=hres.ca |access-date=6 April 2024}}</ref> |
|||
| legal_UK = <!-- GSL, P, POM, CD, or Class A, B, C --> |
| legal_UK = <!-- GSL, P, POM, CD, or Class A, B, C --> |
||
| legal_US = Rx-only |
| legal_US = Rx-only |
||
| legal_status = |
| legal_status = |
||
| routes_of_administration = Topical |
| routes_of_administration = Topical [[Ophthalmic drug administration|eye drops]] |
||
<!--Pharmacokinetic data--> |
<!--Pharmacokinetic data--> |
||
| bioavailability |
| bioavailability = |
||
| protein_bound |
| protein_bound = |
||
| metabolism = Activation by ester [[hydrolysis]], deactivation by [[beta oxidation]] |
|||
| metabolism = |
|||
| metabolites = |
|||
| ⚫ | |||
| onset = 2–4 hrs |
|||
| ⚫ | |||
| ⚫ | |||
| duration_of_action = ≥ 24 hrs |
|||
| ⚫ | |||
<!--Identifiers--> |
<!--Identifiers--> |
||
| Line 31: | Line 36: | ||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
| ChEBI_Ref = {{ebicite|correct|EBI}} |
||
| ChEBI = 66899 |
| ChEBI = 66899 |
||
| DrugBank = |
| DrugBank = DB08819 |
||
| ChEMBL = 1963683 |
| ChEMBL = 1963683 |
||
| ChemSpiderID = 8044182 |
| ChemSpiderID = 8044182 |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
| UNII_Ref = {{fdacite|correct|FDA}} |
||
| UNII = 1O6WQ6T7G3 |
| UNII = 1O6WQ6T7G3 |
||
| KEGG = D06274 |
|||
<!--Chemical data--> |
<!--Chemical data--> |
||
| chemical_formula = |
|||
| C=25 | H=34 | F=2 | O=5 |
| C=25 | H=34 | F=2 | O=5 |
||
| molecular_weight = 452.531266 g/mol |
|||
| smiles = CC(C)OC(=O)CCC\C=C/CC(C(O)CC1O)C1\C=C\C(F)(F)COc2ccccc2 |
| smiles = CC(C)OC(=O)CCC\C=C/CC(C(O)CC1O)C1\C=C\C(F)(F)COc2ccccc2 |
||
| StdInChI = 1S/C25H34F2O5/c1-18(2)32-24(30)13-9-4-3-8-12-20-21(23(29)16-22(20)28)14-15-25(26,27)17-31-19-10-6-5-7-11-19/h3,5-8,10-11,14-15,18,20-23,28-29H,4,9,12-13,16-17H2,1-2H3/b8-3-,15-14+/t20-,21-,22+,23-/m1/s1 |
| StdInChI = 1S/C25H34F2O5/c1-18(2)32-24(30)13-9-4-3-8-12-20-21(23(29)16-22(20)28)14-15-25(26,27)17-31-19-10-6-5-7-11-19/h3,5-8,10-11,14-15,18,20-23,28-29H,4,9,12-13,16-17H2,1-2H3/b8-3-,15-14+/t20-,21-,22+,23-/m1/s1 |
||
| StdInChIKey = WSNODXPBBALQOF-VEJSHDCNSA-N |
|||
}} |
}} |
||
'''Tafluprost''' (trade names '''Taflotan''' |
'''Tafluprost''' (trade names '''Taflotan''' by [[Santen Pharmaceutical]], '''Zioptan''' by Merck in the US and '''Saflutan''' by Mundipharma in Australia) is a [[prostaglandin analogue]]. It is used topically (as [[eye drop]]s) to control the progression of [[open-angle glaucoma]] and in the management of [[ocular hypertension]], alone or in combination with other medication. It reduces [[intraocular pressure]] by increasing the outflow of [[Aqueous humour|aqueous fluid]] from the eyes.<ref name="PPA" /><ref name="AC" /> |
||
==Adverse effects== |
|||
Taflotan contains 15 µg/ml Tafluprost. ''Taflotan sine'' is a preservative-free, single-dose formulation containing 0.3 ml per dose.<ref>[http://www.gelbe-liste.de/pharmindex/praeparat/taflotan-sine-15-mikrogrammml-augentropfen-in-einzeldosen-santen-gmbh/ Gelbe Liste (in German)]</ref> |
|||
The most common side effect is [[conjunctival hyperemia]], which occurs in 4 to 20% of patients. Less common side effects include stinging of the eyes, headache, and [[respiratory infection]]s. Rare side effects are [[dyspnoea]] (breathing difficulties), worsening of [[asthma]], and [[macular oedema]].<ref name="PPA" /><ref name="AC" /><ref name="Dinnendahl" /> |
|||
== |
== Interactions == |
||
| ⚫ | |||
[[Nonsteroidal anti-inflammatory drug]]s (NSAIDs) can either reduce or increase the effect of tafluprost.<ref name="PPA" /> [[Timolol]] eye drops, a common kind of glaucoma medication, does not negatively interact with this drug.<ref name="AC" /> |
|||
No interactions with systemic (for example, oral) drugs are expected because tafluprost does not reach relevant concentrations in the bloodstream.<ref name="AC" /><ref name="Dinnendahl" /> |
|||
==Pharmacology== |
|||
===Mechanism of action=== |
|||
Tafluprost is a [[prodrug]] of the active substance, tafluprost acid, a [[structural analogue|structural]] and [[Functional analog (chemistry)|functional analogue]] of [[prostaglandin F2α|prostaglandin F<sub>2α</sub>]] (PGF<sub>2α</sub>). Tafluprost acid is a selective [[agonist]] at the [[prostaglandin F receptor]], increasing outflow of aqueous fluid from the eyes and thus lowering intraocular pressure.<ref name="AC" /><ref name="Dinnendahl" /> |
|||
Other PGF<sub>2α</sub> analogues with the same mechanism include [[latanoprost]] and [[travoprost]].<ref name="AC" /> |
|||
===Pharmacokinetics=== |
|||
Tafluprost, as a [[lipophilic]] [[ester]], easily penetrates the [[cornea]] and is then activated to the [[carboxylic acid]], tafluprost acid. Onset of action is 2 to 4 hours after application, the maximal effect is reached after 12 hours, and ocular pressure remains lowered for at least 24 hours.<ref name="AC" /><ref name="Dinnendahl" /> |
|||
Tafluprost acid is inactivated by [[beta oxidation]] to 1,2-dinortafluprost acid, 1,2,3,4-tetranortafluprost acid, and its [[lactone]], which are subsequently [[glucuronidated]] or [[hydroxylated]]. The [[cytochrome P450]] liver enzymes play no role in the metabolism.<ref name="Dinnendahl" /> |
|||
An analogous pathway (at least up to the tetranor-metabolites) has been found for latanoprost and travoprost. |
|||
[[File:Tafluprost metabolism.svg|thumb|left|upright=3|'''Metabolism'''. From left to right: tafluprost, tafluprost acid (the [[active metabolite]]), 1,2-dinortafluprost acid, 1,2,3,4-tetranortafluprost acid, 1,2,3,4-tetranortafluprost acid lactone<ref name="Fukano09" /><ref name="Fukano11" />]] |
|||
[[File:TAPCOM combination ophthalmic solution.jpg|thumb|A tafluprost/[[timolol]] combination ophthalmic solution]] |
|||
{{clear left}} |
|||
== References == |
|||
| ⚫ | |||
<ref name="PPA">Tafluprost {{drugs.com|PPA|tafluprost}}.</ref> |
|||
<ref name="AC">{{cite book|title=Austria-Codex| veditors = Haberfeld H |publisher=Österreichischer Apothekerverlag | location=Vienna |year=2015 |language=German}}</ref> |
|||
<ref name="Dinnendahl">{{cite book |title= Arzneistoff-Profile| veditors = Dinnendahl V, Fricke U |publisher=Govi Pharmazeutischer Verlag |location=Eschborn, Germany |date=2011 |edition=25 |volume=9|isbn=978-3-7741-9846-3 |language=German}}</ref> |
|||
<ref name="Fukano09">{{cite journal | vauthors = Fukano Y, Kawazu K | title = Disposition and metabolism of a novel prostanoid antiglaucoma medication, tafluprost, following ocular administration to rats | journal = Drug Metabolism and Disposition | volume = 37 | issue = 8 | pages = 1622–34 | date = August 2009 | pmid = 19477946 | doi = 10.1124/dmd.108.024885 | s2cid = 12425702 }}</ref> |
|||
<ref name="Fukano11">{{cite journal | vauthors = Fukano Y, Kawazu K, Akaishi T, Bezwada P, Pellinen P | title = Metabolism and ocular tissue distribution of an antiglaucoma prostanoid, tafluprost, after ocular instillation to monkeys | journal = Journal of Ocular Pharmacology and Therapeutics | volume = 27 | issue = 3 | pages = 251–9 | date = June 2011 | pmid = 21491995 | doi = 10.1089/jop.2010.0178 }}</ref> |
|||
}} |
|||
{{Prostaglandins}} |
{{Prostaglandins}} |
||
| Line 58: | Line 98: | ||
[[Category:Prostaglandins]] |
[[Category:Prostaglandins]] |
||
[[Category:Secondary alcohols]] |
|||
[[Category:Alkene derivatives]] |
|||
[[Category:Drugs developed by Merck & Co.]] |
|||
{{pharma-stub}} |
|||
[[Category:Carboxylate esters]] |
|||
4. Sethi HS, Dhawan M, Naik MP, Gupta VS. Prostaglandin analogs in glaucoma. Astrocyte 2015;2:126-32. |
|||
[[Category:Isopropyl esters]] |
|||
[[Category:Organofluorides]] |
|||
[[Category:Phenol ethers]] |
|||
[[Category:Ophthalmology drugs]] |
|||
Latest revision as of 06:04, 6 April 2024
 | |
| Clinical data | |
|---|---|
| Trade names | Saflutan, Taflotan, Zioptan |
| AHFS/Drugs.com | Multum Consumer Information |
| Routes of administration | Topical eye drops |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Activation by ester hydrolysis, deactivation by beta oxidation |
| Onset of action | 2–4 hrs |
| Duration of action | ≥ 24 hrs |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.207.745 |
| Chemical and physical data | |
| Formula | C25H34F2O5 |
| Molar mass | 452.539 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tafluprost (trade names Taflotan by Santen Pharmaceutical, Zioptan by Merck in the US and Saflutan by Mundipharma in Australia) is a prostaglandin analogue. It is used topically (as eye drops) to control the progression of open-angle glaucoma and in the management of ocular hypertension, alone or in combination with other medication. It reduces intraocular pressure by increasing the outflow of aqueous fluid from the eyes.[2][3]
Adverse effects[edit]
The most common side effect is conjunctival hyperemia, which occurs in 4 to 20% of patients. Less common side effects include stinging of the eyes, headache, and respiratory infections. Rare side effects are dyspnoea (breathing difficulties), worsening of asthma, and macular oedema.[2][3][4]
Interactions[edit]
Nonsteroidal anti-inflammatory drugs (NSAIDs) can either reduce or increase the effect of tafluprost.[2] Timolol eye drops, a common kind of glaucoma medication, does not negatively interact with this drug.[3]
No interactions with systemic (for example, oral) drugs are expected because tafluprost does not reach relevant concentrations in the bloodstream.[3][4]
Pharmacology[edit]
Mechanism of action[edit]
Tafluprost is a prodrug of the active substance, tafluprost acid, a structural and functional analogue of prostaglandin F2α (PGF2α). Tafluprost acid is a selective agonist at the prostaglandin F receptor, increasing outflow of aqueous fluid from the eyes and thus lowering intraocular pressure.[3][4]
Other PGF2α analogues with the same mechanism include latanoprost and travoprost.[3]
Pharmacokinetics[edit]
Tafluprost, as a lipophilic ester, easily penetrates the cornea and is then activated to the carboxylic acid, tafluprost acid. Onset of action is 2 to 4 hours after application, the maximal effect is reached after 12 hours, and ocular pressure remains lowered for at least 24 hours.[3][4]
Tafluprost acid is inactivated by beta oxidation to 1,2-dinortafluprost acid, 1,2,3,4-tetranortafluprost acid, and its lactone, which are subsequently glucuronidated or hydroxylated. The cytochrome P450 liver enzymes play no role in the metabolism.[4]
An analogous pathway (at least up to the tetranor-metabolites) has been found for latanoprost and travoprost.


References[edit]
- ^ "Product monograph" (PDF). hres.ca. Retrieved 6 April 2024.
- ^ a b c Tafluprost Professional Drug Facts.
- ^ a b c d e f g Haberfeld H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
- ^ a b c d e Dinnendahl V, Fricke U, eds. (2011). Arzneistoff-Profile (in German). Vol. 9 (25 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- ^ Fukano Y, Kawazu K (August 2009). "Disposition and metabolism of a novel prostanoid antiglaucoma medication, tafluprost, following ocular administration to rats". Drug Metabolism and Disposition. 37 (8): 1622–34. doi:10.1124/dmd.108.024885. PMID 19477946. S2CID 12425702.
- ^ Fukano Y, Kawazu K, Akaishi T, Bezwada P, Pellinen P (June 2011). "Metabolism and ocular tissue distribution of an antiglaucoma prostanoid, tafluprost, after ocular instillation to monkeys". Journal of Ocular Pharmacology and Therapeutics. 27 (3): 251–9. doi:10.1089/jop.2010.0178. PMID 21491995.
