Tenoxicam
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
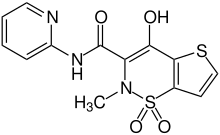
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Tenoxicam | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 13 H 11 N 3 O 4 S 2 | ||||||||||||||||||
| Brief description |
yellow, crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 337.3741 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
211 ° C |
||||||||||||||||||
| pK s value |
4.78 |
||||||||||||||||||
| solubility |
Water: 14.1 mg mL −1 at 25 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tenoxicam is a nonsteroidal anti-inflammatory drug (NSAID) from the group of oxicams with analgesic , antiphlogistic and antipyretic properties. It is intended for oral, rectal and intravenous administration and is used for rheumatoid pain, osteoarthritis , ankylosing spondylitis , tendinitis, ankylosing spondylitis , post-traumatic pain, bursitis , tendinitis , frozen shoulder , strains and sprains or acute gout .
history
It was patented in 1976 by Hoffmann-La Roche , invented by Rudolf Pfister, Paul Zeller, Dieter Binder and Otto Hromatka . In Europe, Tenoxicam was introduced in France in 1982. Clinical trials for use as analgesics began shortly thereafter. In Germany, tenoxicam was sold as an active substance analog to piroxicam between 1987 and 2000 under the names Liman and Tilcotil .
Extraction
In the literature, the production based on a Fiesselmann thiophene synthesis is described.
properties
The keto-enol tautomerism is analogous to the derivative lornoxicam .
Under standard conditions , tenoxicam is a yellow, crystalline powder. Like most nonsteroidal antiinflammatory drugs, it is having a pK s of 4.78, a weak acid.
pharmacology
Pharmacodynamics
Tenoxicam blocks prostaglandin synthesis by inhibiting cyclooxygenases ; it also inhibits platelet aggregation . Studies on cyclooxygenase isoenzymes showed that tenoxicam inhibits cyclooxygenase isoenzymes in the ratio COX-2 / COX-1 of 1.34. Experiments with leukocyte peroxidase show that tenoxicam acts as a radical scavenger at the site of inflammation .
Pharmacokinetics
The active ingredient is metabolized in the liver via cytochrome P450 2C9 with the main, inactive metabolite 5-hydroxy-tenoxicam with absolute bioavailability. The maximum plasma concentrations when taken on an empty stomach can be observed within the first two hours. The volume of distribution in steady state is on average between 10 and 11 liters. Tenoxicam is more than 99% bound to albumin in the blood . Up to two thirds are excreted in the urine, the rest in the bile, mainly in the form of glucuronidated compounds. Less than 1% of the administered dose is excreted unchanged in the urine.
The plasma half-life is between 49 and 81 hours, with an average of 72 hours.
The usual daily dose is taken once a day due to the slow metabolism. Although the therapeutic effect occurs soon after the start of treatment, it continues to increase during the first two weeks until the plasma concentration has reached steady state.
Clinical information
Contraindications
Tenoxicam should not be used in the last trimester of pregnancy. Other contraindications are Crohn's disease , ulcerative colitis , severe liver, heart or kidney dysfunction. Elderly patients, patients with an increased risk of kidney failure and patients with an increased risk of bleeding should not receive the drug before anesthesia or surgery, since, as with all NSAIDs, the risk of acute kidney failure is increased and there is a possibility of hemostasis disorders. Combination treatment with salicylates or other NSAIDs should be avoided because of the increased risk of gastrointestinal side effects.
Too little clinical experience is available for patients under 18 years of age.
Side effects
Stomach pain, heartburn, nausea , diarrhea, or constipation are very common . Pruritus , exanthema , erythema , urticaria , dizziness, headache, dyspepsia , epigastric and abdominal complaints or an increase in creatinine, SGOT, SGPT or bilirubin were also observed in 1–2% . Occasionally there are signs of fatigue, loss of appetite, sleep disorders, vertigo , palpitations, stomatitis , gastritis , vomiting, or dry mouth. Rarely to very rarely may lead to a decrease in hemoglobin levels to anemia , agranulocytosis , granulocytopenia , leukopenia , thrombocytopenia , mild edema, photodermatosis , gastrointestinal bleeding including haematemesis and melena, ulcers, hypersensitivity reactions such as dyspnoea , asthma, anaphylaxis, angioedema , blurred vision, Vasculitis, photosensitivity, or gastrointestinal perforation.
The renal effects of NSAIDs include fluid retention with edema and / or arterial hypertension. Very rarely, serious, sometimes fatal skin reactions, including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, can occur. The use of tenoxicam can affect female fertility and is therefore not recommended in women trying to become pregnant.
Interactions
With concurrent use of diuretics , corticosteroids, anti-coagulants coumarin-type, selective serotonin reuptake inhibitors , platelet inhibitors, or ACE inhibitors caution, concomitant use with other non-steroidal anti-inflammatory drugs, including COX-2 -selective Inhibatoren is to avoid.
Salicylates displace tenoxicam from the protein binding sites, thereby increasing the volume of distribution. Concomitant use of methotrexate has been associated with decreased secretion of renal tubules, higher plasma concentrations, and severe methotrexate toxicity. Plasma levels and toxicity of lithium are increased by simultaneous ingestion.
Trade names
Tenoxicam is commonly sold as a single preparation . Trade names: Dolmen (I), Rexalgan (I), Tilcotil (CH, B, I, J, TR), Tilko (TR), Vienoks (TR), Mobiflex (GB)
See also
Individual evidence
- ↑ a b Entry on tenoxicam. In: Römpp Online . Georg Thieme Verlag, accessed on February 13, 2016.
- ↑ CB2163299 in ChemicalBook , accessed February 2016.
- ↑ D01767 at KEGG , accessed in February 2016.
- ↑ Entry on tenoxicam in the Human Metabolome Database (HMDB) , accessed on 2016-02-19.
- ↑ a b Entry on tenoxicam in the DrugBank of the University of Alberta .
- ↑ a b Tenoxicam data sheet from Sigma-Aldrich , accessed on February 13, 2016 ( PDF ).
- ↑ a b c d e f g Entry on tenoxicam in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on April 11, 2016.
- ↑ a b c d Tilcotil in the Swiss Medicines Compendium, accessed in February 2016.
- ↑ patent application DE2537070 : thiazines. Registered on August 20, 1975 , published on March 18, 1976 , applicant: Hoffmann-La Roche Inc., inventors: Rudolf Pfister, Paul Zeller, Dieter Binder, Otto Hromatka.
- ^ D Penso: Toxic epidermal necrolysis after oxicam use. . In: Journal of the American Academy of Dermatology . 14, 1986, pp. 275-6. doi : 10.1016 / s0190-9622 (86) 80342-5 . PMID 3485122 .
- ^ Institute for Health and Social Research GmbH: Analog active ingredients in the drug market: Therapeutic use and importance for health insurance expenses (PDF), Structural Research in Health Care Volume 30, pp. 138f, Berlin 2001. ISBN 3-9808407-1-9 . Retrieved February 26, 2016.
- ↑ Dieter Binder, Otto Hromatka, Franz Geissler, Karl Schmied, Christian R. Noe, Kaspar Burri, Rudolf Pfister, Konrad Strub, Paul Zeller: Analogs and derivatives of tenoxicam. 1. Synthesis and anti-inflammatory activities of analogs with different residues on the ring nitrogen and the amide nitrogen . In: . Journal Med. Chem . April 30, 1987, pp. 678-682. doi : 10.1021 / jm00387a017 .
- ↑ D. Binder, O. Hromatka, F. Geissler, K. Schmied, CR Noe, K. Burri, R. Pfister, K. Strub, P. Zeller: Analogues and derivatives of tenoxicam. 1. Synthesis and anti-inflammatory activity of analogues with different residues on the ring nitrogen and the amide nitrogen. In: Journal of medicinal chemistry. Volume 30, Number 4, April 1987, pp. 678-682, PMID 3494124 .
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher and Dietmar Reichert: Pharmaceutical Substances, 4th edition (2000), 2 volumes published by Thieme-Verlag Stuttgart, ISBN 978-1-58890-031-9 ; online since 2003 with biannual additions and updates.
- ^ OG Nilsen: Clinical Pharmacokinetics of tenoxicam . In: Clin Pharmacokinet . 1, 1996, pp. 16-43. PMID 8137596 .
- ↑ a b Drugs.com International: Tenoxicam (English).
