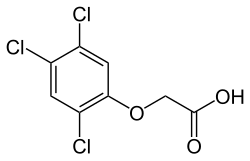
2,4,5-trichlorophenoxyacetic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,4,5-trichlorophenoxyacetic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 5 Cl 3 O 3 | ||||||||||||||||||
| Brief description |
colorless and odorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 255.48 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.80 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
153 ° C |
||||||||||||||||||
| boiling point |
decomposition |
||||||||||||||||||
| solubility |
very bad in water (278 mg l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
|
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2,4,5-Trichlorophenoxyacetic acid ( 2,4,5-T for short , also known as T-acid ) is a herbicide derived from phenoxyacetic acid . 2,4,5-T was of environmental relevance primarily due to its contamination with 2,3,7,8-tetrachlorodibenzodioxin (2,3,7,8-TCDD). It was an important part of the defoliants used in the Vietnam War . Accidents during production led to the release of dioxins a few times.
history
2,4,5-T came on the market in 1948. Serious accidents involving the release of dioxins repeatedly occurred during its manufacture . In an industrial accident at the Monsanto Nitro production facility in 1949, over 120 workers were contaminated with the reaction mixture and contracted chloracne . Another serious accident occurred in 1952 at Boehringer Ingelheim in Hamburg-Moorfleet , after which 30 workers fell ill with chloracne. During investigations to optimize the 2,4,5-T synthesis in 1968 a reaction vessel exploded in the laboratory of the British Coalite Chemicals, killing a worker. The laboratory building was initially continued to be used, but this led to the occurrence of chloracne in around 80 employees.
At the time of the Vietnam War, 2,4,5-T, partly in the form of its iso- or n- butyl esters , was the active ingredient of the defoliants Agent Orange , Agent Green , Agent Pink and Agent Purple . These agents contained between 0.02 and 15 ppm (mg / kg) 2,3,7,8-TCDD, which was almost exclusively due to production-related impurities in the 2,4,5-trichlorophenoxyacetic acid used. The amount of 2,3,7,8-TCDD released during the Vietnam War was estimated to be around 166 kg.
Between 1971 and 1974, a specialist Missouri company improperly disposed of oily residues from 2,4,5-T production. Instead, she sprayed them on horse races to collect the dust. Horses are very sensitive to dioxin, more than 50 animals died as a result. These debris had also been spread on the streets of Times Beach . During a flood in 1982, the dioxin-containing road surface was washed into houses. The entire place was bought by the state and the population was resettled.
Mode of action and use
2,4,5-T has a similar effect to the structurally related 2,4-dichlorophenoxyacetic acid like the plant growth hormone auxin . It triggers overgrowth, which quickly leads to the death of the plant.
2,4,5-T was used in the 1970s and 1980s under the name Tormona (e.g. Tormona 80, Tormona 100), particularly against woody plants and for so-called purification in the forest. In combination with other herbicides, it was also used in grain cultivation and on grassland and lawns. The herbicide was mostly sold in the form of its water-soluble alkali or amine salts as a powder, the 2,4,5-T esters as emulsion concentrates. The use of 2,4,5-T has been banned in Germany since 1988. In Austria and Switzerland, too, there is no longer any approval as a plant protection product.
Manufacturing
For the preparation, tetrachlorobenzene was converted into 2,4,5-trichlorophenol , which was then converted into 2,4,5-trichlorophenoxyacetic acid at about 140 ° C. with chloroacetic acid . If the temperature rose too much during this last reaction, dioxins were formed.
toxicology
2,4,5-T is mainly absorbed through the skin or the digestive tract. It has a strong irritant effect on the mucous membranes and skin , and this effect is mainly attributed to traces of 2,3,7,8-TCDD. The general condition may be disturbed after admission. The substance damages the nervous and cardiovascular systems. No clinical symptoms occurred in volunteers who had swallowed 2,4,5-T at doses up to 5 mg / kg body weight. All they reported was a metallic taste in the mouth. In animal experiments, the lethal dose (LD 50 ) was 100 mg / kg body weight in dogs, 500 mg / kg in rats and about 800 mg / kg in mice. In the studies carried out on chronic toxicity, it seems in most cases no longer to be traceable to what extent the 2,4,5-T used was contaminated with dioxins. In animal experiments, a NOAEL of 3 mg 2,4,5-T / kg body weight was determined. The chronic effects caused damage to the liver and kidneys. The permitted daily dose for humans was set at 0.03 mg 2,4,5-T / kg body weight. Pure 2,4,5-trichlorophenoxyacetic acid is probably not teratogenic , mutagenic or carcinogenic .
On average, 2,4,5-T contained about 10 ppm of dioxins. For a long time, Germany had a limit value for 2,3,7,8-TCDD in 2,4,5-T products of 10 mg / kg. It was gradually reduced; in the 1980s, 2,4,5-T was allowed to contain a maximum of 0.1 mg / kg TCDD.
Environmental impact
The compound was decomposed in plants after the acetic acid residue had been split off by hydroxylation on the ring. The post-action time in the soil after the application of 1.5 kg 2,4,5-T per hectare is given as about 2 months. 2,4,5-T was not classified as dangerous for bees . The LC50 for rainbow trout was determined for a 2,4,5-T-ester at 12 mg in a 24-hour test.
proof
For residue analysis , 2,4,5-trichlorophenoxyacetic acid can be extracted with chloroform and, after purification of the extract, converted into trichlorophenol with the aid of pyridine hydrochloride . The trichlorophenol can be determined colorimetrically after reaction with 4-amino antipyrine and potassium ferricyanide . Alternatively, the purified 2,4,5-T extract can also be esterified with dimethyl sulfate and determined with the aid of gas chromatography .
Individual evidence
- ↑ a b c d e f g h i j k Entry on 2,4,5-trichlorophenoxyacetic acid in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on 2,4,5-T in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 93-76-5 or 2,4,5-trichlorophenoxyacetic acid ), accessed on November 2, 2015.
- ↑ Entry on 2,4,5-trichlorophenoxyacetic acid in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b c John Emsley: Dioxins, the deadliest poisons in the world? In: Parfum, Portwein, PVC… Wiley-VCH, Weinheim 2003, ISBN 3-527-30789-3 .
- ↑ Dieter Lenoir, Heinrich Sandermann Jr .: Origin and Effects of Dioxins , Biology in our Time , 23 (6), pp. 363-369 (1993), ISSN 0045-205X .
- ↑ a b c d e Werner Perkow: Active substances in pesticides and pesticides. 2nd edition, Paul Parey publishing house.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on 2,4,5-T in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; Retrieved March 3, 2016.
- ↑ Karlheinz Ballschmiter, Reiner Bacher: Dioxins. VCH, Weinheim 1996, ISBN 3-527-28768-X .

