2,4,6-tri- tert -butylphenol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2,4,6-tri- tert -butylphenol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 18 H 30 O | |||||||||||||||
| Brief description |
Crystalline, yellow solid or pale yellow crystal powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 262.44 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.864 g cm −3 (27 ° C ) |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
|
|||||||||||||||
| pK s value |
12.2 |
|||||||||||||||
| solubility |
very slightly soluble in water (35 μg cm −3 at 2 ° C ) and alkali, soluble in organic solvents, such as B. ethanol , acetone and carbon tetrachloride |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
2,4,6-tri- tert -butylphenol (2,4,6-TTBP) is a three tert -butyl groups symmetrically substituted, and thereby greatly hindered phenol . The high degree of substitution makes 2,4,6-TTBP an easily oxidizable, electron-rich aromatic and a very weak acid. The deep blue 2,4,6-tri- tert- butylphenoxy radical produced by oxidation is stable for several weeks at room temperature.
In contrast to other alkylphenols , the compound is practically insoluble in alkaline media and also does not show the characteristic coloration of phenols with iron (III) chloride . 2,4,6-TTBP is used as an antioxidant in industrial applications.
Occurrence and representation
1890 received Wilhelm Koenigs in the reaction of phenol with a branched alkene isoamylene ( isopentene , 2-methyl-2-butene), and catalytic amounts of concentrated sulfuric acid complex mixtures of unknown composition - presumably from Phenylalkylethern and alkylphenols - and correctly concluded that "in In this case there appears to be a direct addition of an unsaturated hydrocarbon to an aromatic substance ”.
Further work showed that in the electrophilic aromatic substitution of phenol with isobutene (2-methyl-2-propene) in the presence of Friedel-Crafts catalysts , such as. B. aluminum chloride , boron trifluoride , acidic ion exchangers or aluminum phenolates product mixtures are formed which, in addition to relatively little 2,4,6-TTBP, the monosubstitution products 2- tert -butylphenol (2-TBP) and 4- tert -butylphenol (4-TBP), as well as the Disubstitution products 2,4-di- tert -butylphenol (2,4-DTBP), 2,6-di-tert-butylphenol (2,6-DTBP) and 2,5-di- tert -butylphenol (2,5-DTB ) contain.
Even under mild reaction conditions, such as. B. with tert- butanol as a reactant and the ionic liquid TEBSA-HSO 4 (from 1,4- butane sultone , triethylamine and sulfuric acid) as a catalyst and solvent - conditions that are classified as green chemistry - result in product mixtures that in this case predominantly contain 2,4-DTBP.
The low-substituted phenol derivatives can be isolated from the product mixtures by distillation. The 2,4,6-TTBP can be separated from the by-product 2,5-DTBP, which is difficult to separate, by melt crystallization in a very high purity (> 99.9%).
2,4,6-tri- tert- butylphenol is also obtained as a (mostly undesirable) by-product in the synthesis of the disubstitution products 2,4-DTBP and 2,6-DTBP, which are used in larger amounts as antioxidants.
The targeted synthesis of 2,4,6-tri- tert- butylphenol, which is also suitable as a practical experiment due to its simplicity - only liquid reactants - is based on phenol, excess methyl- tert-butyl ether (MTBE) as the alkylating agent (via the tertiary carbenium ion formed as an intermediate ) and sulfuric acid as a catalyst, 2,4,6-TTBP being obtained in 69% yield.
Phenol reacts at temperatures of 50-60 ° C in the presence of acidic catalysts, such as. B. sulfuric acid or aluminum chloride and an excess of gaseous isobutene in yields of up to 90% to 2,4,6-tri- tert- butylphenol.
properties
2,4,6-Tri- tert -butylphenol is a yellowish solid that dissolves in many organic solvents, but not in aqueous or alcoholic alkali solutions. The green-blue coloration with iron (III) chloride, which is characteristic of phenols, does not occur with 2,4,6-TTBP. The compound is oxidizable in the air, but practically not biodegradable.
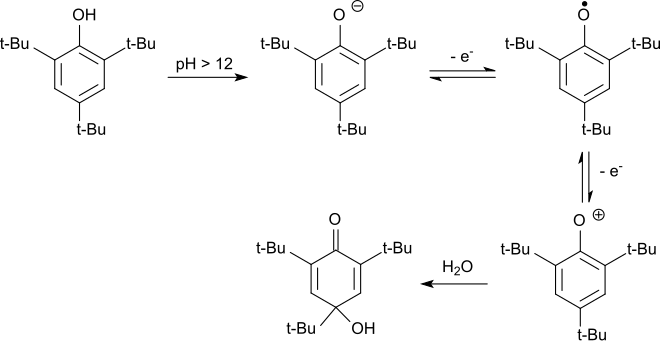
As an electron-rich aromatic, 2,4,6-tri- tert- butylphenol can also be easily oxidized electrochemically. In the alkaline environment, the phenolate anion formed is first oxidized to the phenoxy radical in a reversible reaction. The stable radical is oxidized to the phenoxonium cation by further electron withdrawal, which reacts in water to form 2,4,6-tri- tert- butyl-4-hydroxy-2,5-cyclohexadienone.
In acidic media, the hydroxydienone is dealkylated with cleavage of the tert-butyl group in the 4-position to give 2,6-di- tert -butylhydroquinone, which is oxidized to the end product 2,6-di- tert- butyl-1,4-benzoquinone.
The oxidation of 2,4,6-tri- tert- butylphenol in the alkaline to the intensely blue colored phenoxy radical can also take place with potassium hexacyanidoferrate (III) . The 2,4,6-tri- tert- butylphenoxy radical forms blue crystals on cooling to −70 ° C, which are stable for several weeks at room temperature and only gradually turn yellow. The phenoxy radical reacts with atmospheric oxygen as a diradical to form a 4,4'-linked peroxide, which forms yellow crystals.
Applications
The electron-rich 2,4,6-tri- tert- butylphenol can easily be oxidized to the phenoxy radical, which in the 4-position adds phenols, as well as alcohols and thiols, to the corresponding cyclohexadienones. The cyclohexadienones, also referred to in the literature as quinol ethers, split off the 4-position tert- butyl group when heated in acid and aromatize to give the substituted phenol.
The reaction can be used for the synthesis of 2,6-di- tert- butyl-4-methoxyphenol, which is frequently used as an antioxidant .
2,4,6-Tri- tert -butylphenol, like the other polyalkylated alkylphenols, is used as stabilizers , free radical scavengers and antioxidants in technical applications, e.g. B. used in fuels , hydraulic fluids and lubricating oils , as well as in elastomeric and thermoplastics . Due to its pronounced persistence , its high tendency to bioaccumulate and aquatic toxicity , 2,4,6-TTBP is only used in a limited way in industry and is e.g. B. Banned in Japan.
The phenoxy radical from 2,4,6-TTBP is also described as a sterically demanding protecting group in a reagent for the transfer of a nucleophilic dimethylaminomethyl - [(CH 3 ) 2 N-CH 2 -] group with the formation of tertiary amines.
Safety instructions / toxicology
In 2017, 2,4,6-tri- tert- butylphenol was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of 2,4,6-tri- tert- butylphenol were concerns about environmental exposure and the dangers arising from a possible assignment to the group of PBT / vPvB substances. The reassessment took place from 2017 and was carried out by Belgium . A final report was then published.
Web links
Individual evidence
- ↑ a b c d e data sheet 2,4,6-tri-tert-butylphenol from Sigma-Aldrich , accessed on January 6, 2017 ( PDF ).
- ↑ a b c d Entry on 2,4,6-tri-tert-butylphenol at TCI Europe, accessed on January 6, 2017.
- ^ A b William M. Haynes: CRC Handbook of Chemistry and Physics, 94th Edition . CRC Press, Boca Raton, FL, USA 2015, ISBN 978-1-4665-7114-3 , pp. 3-518 .
- ↑ a b G.H. Stillson, DW Sawyer, CK Hunt: The hindered phenols . In: J. Am. Chem. Soc. tape 67 , no. 2 , 1945, p. 303-307 , doi : 10.1021 / ja01218a045 .
- ↑ a b c Screening Assessment for the Challenge, Phenol, 2,4,6-tris (1,1-dimethylethyl) - (2,4,6-tri-tert-butylphenol), CAS Registry Number 732-26-3. Environment Canada, Health Canada, November 2008, accessed January 11, 2017 .
- ↑ a b C.D. Cook, DA Kuhn, P. Fianu: Oxidation of hindered phenols. IV. Stable phenoxy radicals . In: J. Am. Chem. Soc. tape 78 , no. 9 , 1956, pp. 2002–2005 , doi : 10.1021 / ja01590a067 .
- ↑ W. Koenigs: About condensations of unsaturated hydrocarbons with phenols . In: Chem. Ber. tape 23 , no. 2 , 1890, p. 3144-3146 , doi : 10.1002 / cber.189002302257 .
- ↑ Patent US3133974 : phenol alkylation process. Applied on November 23, 1960 , published May 19, 1964 , Applicant: Ethyl Corp., Inventor: RP Curry, JC Geddes.
- ↑ AR Hajipour, Y. Ghayeb, N. Sheikhan, AE Ruoho: Brønsted acidic ionic liquid as an efficient and reusable catalyst for one-pot synthesis of 1-amidoalkyl 2-naphthols under solvent-free conditions . In: Tetrahedron Lett. tape 50 , 2009, p. 5649-5651 , doi : 10.1016 / j.tetlet.2009.07.116 .
- ↑ P. Elavarasan, K. Kondamundi, S. Upadhyayula: Synthesis of antioxidants: green chemistry route . In: Int. J. Chem. Sci. tape 8 , no. 5 , 2010, p. S578-S584 ( academia.edu ).
- ↑ Patent WO0014043 : Purification of alkylated phenols by melt crystallization. Filed July 1, 1999 , published March 16, 2000 , applicant: General Electric Co., inventor: AJ Mahood.
- ↑ a b B.G. Somers, CD Cook: The preparation of 2,4,6-tri-tert-butylphenol . In: J. Chem. Educ. tape 32 , no. 6 , 1955, pp. 312 , doi : 10.1021 / ed032p312 .
- ^ JA Richards, PE Whitson, DH Evans: Electrochemical oxidation of 2,4,6-tri-tert-butylphenol . In: J. Electroanal. Chem. Band 63 , no. 3 , 1975, p. 311-327 , doi : 10.1016 / s0022-0728 (75) 80303-2 .
- ^ CD Cook, RC Woodworth: Oxidation of hindered phenols. II. The 2,4,6-tri-t-butylphenoxy radical . In: J. Am. Chem. Soc. tape 75 , no. 24 , 1953, pp. 6242-6244 , doi : 10.1021 / ja01120a040 .
- ^ E. Müller, K. Ley, G. Schlechte: About Oxygen Radicals, VIII. About Dehydration of Phenols . In: Chem. Ber. tape 90 , no. 11 , 1957, pp. 2660-2672 , doi : 10.1002 / cber.19570901136 .
- ↑ Patent US3410878 : Preparation of quinol ethers. Applied on April 1, 1964 , published November 12, 1968 , applicant: General Electric Co., inventor: H.-D. Becker.
- ↑ Patent US3895069 : Process for the preparation of 2,4,6-trialkyl-4-alkylthio or 4-alkoxycyclohexadi-2,5-ene-1-ones. Applied February 20, 1973 , published July 15, 1975 , Applicant: Imperial Chemical Industries Ltd., Inventor: JH Atkinson, D. Clark.
- ↑ D. Seebach , T. Hassel: 2,4,6-tri- tert- butylphenoxy (TBPO) as a sterically active carbonyl protective group - a new nucleophilic dimethylaminomethylating agent . In: Angew. Chem. Band 90 , no. 4 , 1978, p. 296-297 , doi : 10.1002 / anie.19780900422 .
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): 2,4,6-tri-tert-butylphenol , accessed on March 26, 2019.