MTBE
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Methyl tert-butyl ether | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 12 O | |||||||||||||||
| Brief description |
colorless liquid with a characteristic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 88.15 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.74 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−109 ° C |
|||||||||||||||
| boiling point |
55 ° C |
|||||||||||||||
| Vapor pressure |
270 hPa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.3664 (25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
DFG / Switzerland: 50 ml m −3 or 180 mg m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
MTBE (according to IUPAC nomenclature: 2-methoxy-2-methylpropane , also spelled out methyl- tert-butyl ether ) is an organic-chemical compound from the group of aliphatic ethers . On the one hand, because of its use as an additive in petrol and, on the other hand, as a solvent in organic chemistry, it has achieved a certain industrial importance.
Extraction and presentation
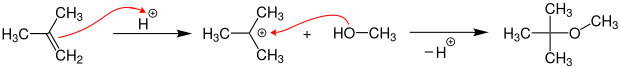
MTBE is produced on an industrial scale by the acid-catalyzed etherification of isobutene with methanol at temperatures of 40–90 ° C. and pressures of 3–20 bar on acidic ion exchange resins .
Physical Properties
MTBE is a colorless, typically ether-like smelling liquid. The compound boils at 55 ° C. under normal pressure. The melting point is −109 ° C. Miscibility with water is limited: a maximum of 4.8 g of MTBE dissolves in 100 g of water at room temperature, conversely, a maximum of 1.4 g of water dissolves in 100 g of MTBE. The azeotrope with water boils at normal pressure at 52.9 ° C. with an azeotrope composition of 96.5% MTBE and 3.5% water (in each case by mass ). An azeotrope boiling at 51 ° C. is formed with methanol at a content of 10% by mass.
Thermodynamic properties
The vapor pressure function results according to Antoine according to log 10 (P) = A− (B / (T + C)) (P in kPa, T in K) with A = 6.34991, B = 1312.52 and C = −26 , 03 in the temperature range from 315 K to 365 K.
The most important thermodynamic properties are listed in the following table:
| property | Type | Value [unit] |
|---|---|---|
| Standard enthalpy of formation | Δ f H 0 liquid Δ f H 0 gas |
−315.4 kJ mol −1 −285.0 kJ mol −1 |
| Standard entropy | S 0 liquid S 0 g |
265.3 J mol −1 K −1 as a liquid 357.8 J mol −1 K −1 as a gas |
| Enthalpy of combustion | Δ c H 0 liquid | −3368.97 kJ mol −1 |
| calorific value | Hu | 35 MJ / kg |
| Heat capacity | c p | 187.5 J mol −1 K −1 (25 ° C) as a liquid |
| Enthalpy of fusion | Δ f H 0 | 7.6 kJ mol −1 at the melting point |
| Entropy of fusion | Δ f S 0 | 46.18 kJ mol −1 at the melting point |
| Enthalpy of evaporation | Δ V H 0 | 27.94 kJ mol −1 at normal pressure boiling point |
| Critical temperature | T C | 223.25 ° C |
| Critical pressure | P C | 33.97 bar |
The temperature dependence of the enthalpy of vaporization can be calculated according to the equation Δ V H 0 = A exp (−β T r ) (1 − T r ) β (Δ V H 0 in kJ / mol, T r = (T / T c ) reduced temperature) with A = 46.23 kJ / mol, β = 0.2893 and T c = 497.1 K in the temperature range between 298 K and 343 K.
Temperature dependence of the heat of vaporization of MTBE
Safety-related parameters
MTBE forms highly flammable vapor-air mixtures. The compound has a flash point at −28 ° C. The explosion range is between 1.6% by volume (58 g / m 3 ) as the lower explosion limit (LEL) and 8.4% by volume (310 g / m 3 ) as the upper explosion limit (UEL). A correlation of the explosion limits with the vapor pressure function results in a lower explosion point of −33 ° C and an upper explosion point of −5 ° C. The limit gap width was determined to be 1 mm. This results in an assignment to explosion group IIA. The ignition temperature is 460 ° C. The substance therefore falls into temperature class T1. The electrical conductivity is rather low at 3.0 · 10 −11 S · m −1 .
According to the dangerous goods regulations , MTBE is assigned to class 3 (flammable liquids) with packaging group II (medium hazard) (label: 3).
use
| MTBE | |
|---|---|
| Brief description | Anti-knock agents for petrol |
| properties | |
| Physical state | liquid |
| Octane number |
118 RON, 101 MOZ |
| Flash point |
−28 ° C |
| Ignition temperature | 460 ° C |
| Explosive limit | 1.6-8.4% by volume |
| Temperature class | T1 |
| safety instructions | |
| UN number | 2398 |
| Hazard number | 33 |
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |
MTBE is mainly used as an anti-knock agent in petrol . It increases the octane number and reduces the tendency of the gasoline engine to knock . It replaces tetraethyl lead in unleaded fuels.
It is also contained in start-up sprays, which are injected into the intake tract of the internal combustion engine in the event of starting difficulties .
MTBE is increasingly being used in organic chemistry as a solvent and extractant. It replaces ethers such as diethyl ether or tetrahydrofuran , since MTBE has a very low to no tendency to form peroxides through auto-oxidation . This is due to the fact that no peroxide can be formed on the tert- butyl side at the α- carbon atom (since it does not carry a hydrogen atom as a tertiary carbon atom) and peroxide formation at the methyl group would have to proceed mechanistically via a very unstable, primary radical .
Environmental sustainability
The environmental compatibility of MTBE is discussed, especially with regard to groundwater . In the USA , drinking water was contaminated due to tank leaks. The admixture is therefore banned in several US states and has been replaced there by tert -amyl methyl ether (TAME). The groundwater hazard from fuel storage at filling stations is z. B. in Germany considered as subordinate, since double-walled underground tanks are mostly used here at the filling stations. MTBE can be noticed in small traces due to the intense smell. At present, ETBE (made from bioethanol and isobutene) is being used more and more in Germany in order to be able to meet the required bio- admixture quotas (see also bioethanol ).
Safety instructions and risk assessment
Because of its high flammability, tert-butyl methyl ether should always be used under a hood . The skin should be protected against splashes by wearing a coat and suitable protective gloves. When filling larger quantities, it is advisable to take measures against electrostatic charging (use of metal cans and funnels that are earthed).
- In the event of inhalation: remove affected person to fresh air immediately. The vapors can cause headaches, drowsiness or fainting.
- In case of skin contact: wash off with plenty of water and soap. Intensive skin contact can lead to the same symptoms as inhalation.
- In the event of eye contact: rinse thoroughly with water ( eye shower ). Call a doctor.
- If swallowed: rinse mouth with plenty of water. Call a doctor.
In 2012, MTBE was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of MTBE were concerns about high (aggregated) tonnage and widespread use and as a potential endocrine disruptor . The re-evaluation has been running since 2014 and is carried out by France . In order to be able to reach a final assessment, further information was requested.
literature
- Volker Linnemann (2003): Environmental behavior of MTBE after groundwater contamination . Writings from Forschungszentrum Jülich, Umwelt / Environment Volume 40 series, ISSN 1433-5530 , ISBN 3-89336-339-4 .
- Günter Pahlke, Heike Leonhardt, Matthias Tappe: Possible environmental pollution from the use of MTBE (methyl tertiary butyl ether) as a fuel additive in Germany and Western Europe , Federal Environment Agency Berlin in the magazine "ERDÖL ERGAS KOHLE", issue 10, 2000.
Web links
Individual evidence
- ↑ a b c d e f g h i j k l m n o Entry on methyl tert-butyl ether in the GESTIS substance database of the IFA , accessed on March 8, 2017(JavaScript required) .
- ↑ Entry on tert-butyl methyl ether. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-344.
- ↑ Entry on tert-butyl methyl ether in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 1634-04-4 or MTBE ), accessed on November 2, 2015.
- ↑ Dr. Bernhard Schleppinghoff, Dipl.-Ing. Martin Becker: Process for the production of methyl tert-butyl ether (MTBE). In: European Patent Office. EC Erdölchemie GmbH, June 29, 1983, accessed on February 13, 2019 .
- ↑ a b c d K. Watanabe, N. Yamagiwa, Y. Torisawa: "Cyclopentyl Methyl Ether as a New and Alternative Process Solvent", in: Org Process Res Dev . 2007 , 11 , pp. 251-258; doi: 10.1021 / op0680136 .
- ^ IM Smallwood: Handbook of organic solvent properties , Arnold London 1996, ISBN 0-340-64578-4 , pp. 210-211.
- ↑ A. Aucejo, S. Lora, R. Munoz, "Isobaric Vapor-Liquid Equilibrium in the system 2-methylpentanes methyl + 1,1-dimethylethyl ether, ethyl + 1,1-dimethylethyl ether, and methyl + 1,1 Dimethylpropyl Ether ", in: J. Chem. Eng. Data , 1998 , 43 , pp. 973-977; doi: 10.1021 / je980090b .
- ↑ a b Arntz, H .; Gottlieb, K .: "High-pressure heat-flow calorimeter determination of the enthalpy of reaction for the synthesis of methyl t-butyl ether from methanol and 2-methylpropene", in: J. Chem. Thermodyn. , 1985 , 17 , pp. 967-972; doi: 10.1016 / 0021-9614 (85) 90009-6 .
- ↑ a b c Andon, RJL; Martin, JF: "Thermodynamic properties of organic oxygen compounds. 40. Heat capacity and entropy of six ethers", in: J. Chem. Thermodynam. , 1975 , 7 , pp. 593-606; doi: 10.1016 / 0021-9614 (75) 90194-9 .
- ↑ Fenwick, JO; Harrop, D .; Head, AJ: "Thermodynamic properties of organic oxygen compounds. 41. Enthalpies of formation of eight ethers", in: J. Chem. Thermodyn. , 1975 , 7 , pp. 943-954; doi: 10.1016 / 0021-9614 (75) 90158-5 .
- ↑ Archived copy ( memento of the original from August 13, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ a b Martin, JF; Andon, RJL: "Thermodynamic properties of organic oxygen compounds. Part LII. Molar heat capacity of ethanoic, propanoic, and butanoic acids", in: J. Chem. Thermodynam. , 1982 , 14 , pp. 679-688; doi: 10.1016 / 0021-9614 (82) 90083-0 .
- ↑ a b Majer, V .; Svoboda, V .: "Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation", Blackwell Scientific Publications, Oxford, 1985, p. 300.
- ↑ a b Daubert, TE; Jalowka, JW; Goren, V .: "Vapor pressure of 22 pure industrial chemicals", in: AIChE Symp. Ser., 1987, 83, 256, pp. 128-156.
- ^ E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ a b François GARIN: FORMULATIONS OF FUELS ( Memento of 15 October 2005 at the Internet Archive ) (PPT).
- ↑ EPA, Gasoline Composition Regulations Affecting LUST Sites (PDF; 1.1 MB)
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Tert-butyl methyl ether , accessed on March 26, 2019.