1,4-butane sultone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,4-butane sultone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 8 O 3 S | |||||||||||||||
| Brief description |
viscous, colorless or clear, light brown liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 136.17 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.34 g cm −3 at 20 ° C |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
|
|||||||||||||||
| solubility |
soluble in water (54 g · cm −3 at 20 ° C ) with decomposition, soluble in organic solvents, e.g. B. methanol and ethanol with gradual reaction, chloroform , diethyl ether and aromatics , such as. B. benzene , toluene and anisole |
|||||||||||||||
| Refractive index |
1.4619-14625 (20 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1,4-butanesultone is a six-membered δ- sultone and the cyclic ester of 4-hydroxybutanesulfonic acid. As a so-called sulfoalkylating agent, 1,4-butane sultone is used to introduce the sulfobutyl group (- (CH 2 ) 4 - SO 3 - ) into hydrophobic compounds with nucleophilic functional groups, e.g. B. hydroxyl groups as in β-cyclodextrin , or amino groups as in polymethine dyes.
The sulfobutyl group is present in it as a neutral sodium salt and considerably increases the water solubility of the derivatives.
Occurrence and representation
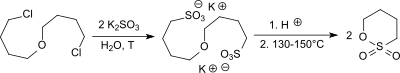
The laboratory synthesis of 1,4-butanesultone starts from 4,4'-dichloro-dibutyl ether (from tetrahydrofuran with phosphorus oxychloride and concentrated sulfuric acid ), which is reacted with sodium sulfite to give the corresponding disodium 4,4'-butanedisulfonic acid salt. By passing through an acidic ion exchanger , the disodium salt is converted into disulphonic acid, which forms two molecules of 1,4-butane sultone at elevated temperature and reduced pressure with elimination of water. The yields achieved are 72 to 80%.
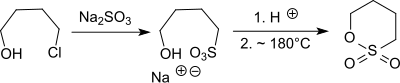
Starting from 4-chlorobutan-1-ol (from tetrahydrofuran and hydrogen chloride in 54 to 57% yield), the sodium salt of 4-hydroxybutane-1-sulfonic acid is obtained with sodium sulfite. The salt is in turn with strong acids, such as. B. hydrochloric acid converted into the very hygroscopic 4-hydroxybutanesulfonic acid and cyclized with elimination of water to give 1,4-butanesultone.
The cyclization of 4-hydroxybutane-sulfonic acid in aqueous solution is particularly efficient when heated with high-boiling and water-immiscible solvents, such as. B. 1,2-dichlorobenzene or diethylbenzene , in which 1,4-butane sultone dissolves and is thereby withdrawn from hydrolysis in an aqueous medium. At the boiling point of the solvent (approx. 180 ° C.) 1,4-butane sultone is obtained in yields of up to 99% within one hour.
The vacuum distillation of the sodium salt of 4-hydroxybutane sulfonic acid in the presence of concentrated sulfuric acid also leads directly to 1,4-butane sultone.
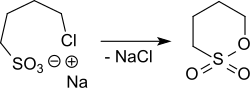
The sodium salt of 4-chlorobutane-1-sulfonic acid, which is accessible from 1,4-dichlorobutane with sodium sulfite, can also be cyclized to 1,4-butane sultone by heating to 180-250 ° C.
The radical-initiated sulfochlorination of 1-chlorobutane leads to a mixture of positionally isomeric sulfochlorides and chlorination products and is not suitable for the targeted preparation of 1,4-butane sultone.
properties
1,4-Butane sultone is a viscous, clear, colorless and odorless liquid that reacts in boiling water (to form 4-hydroxybutane sulfonic acid) and alcohols (to form 4-alkoxybutane sulfonic acid) and dissolves in many organic solvents. At temperatures below the melting point, the compound crystallizes in "large, magnificent plates". Compared to the homologous γ-sultone 1,3-propane sultone , 1,4-butane sultone is significantly less reactive as an alkylating agent, but rated as mutagenic and carcinogenic .
Applications
Sulfobetaines
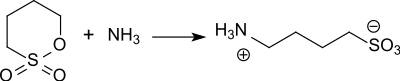
1,4-butane sultone reacts with nucleophiles , such as. B. ammonia with ring opening smoothly to the corresponding zwitterionic , usually very water-soluble sulfobutyl betaines.
Sulfobetaines with longer alkyl chains C n H 2n + 1 with n> 10 show interesting properties as surface-active compounds ( surfactants , detergents ) with antimicrobial properties.
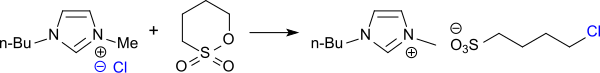
The reaction of N - n -butylimidazole with 1,4-butane sultone in toluene produces 1-butylimidazolium-3- (n-butylsulfonate) in 98% yield
as a component of multifunctional catalysts, the implementation of platform chemicals from biomass , such. B. levulinic acid or itaconic acid is catalyzed into the corresponding lactones , diols or cyclic ethers .
Aminoalkylphosphonic acids, such as. B. Aminomethanediphosphonic acid (from phosphorus trichloride , formamide and phosphonic acid ) forms N- (sulfobutyl) aminomethanediphosphonic acid with 1,4-butane sultone,
which is characterized by its very high solubility in water (<1000 g · l −1 ) and distinctive properties as a complexing agent and water softener .
Sulfobutylation of cyanine dyes leads to compounds that are readily soluble in water and that interact with proteins such as. B. Link antibodies and use as pH-sensitive fluorescent markers.
Ionic liquids
The ionic liquid 4-triethylammoniumbutane-1-sulfonic acid hydrogen sulfate (TEBSA HSO4) is formed from the reaction of 1,4-butane sultone with triethylamine in acetonitrile to form the zwitterion (85% yield) and subsequent reaction with concentrated sulfuric acid,
which can replace conventional mineral acids as effective and easily recyclable acid catalysts in solvent-free reactions.
The ring opening of 1,4-butane sultone with organic chloride salts gives ionic liquids of the 4-chlorobutylsulfonate type in quantitative yield.
The chlorine atom in the 4-chlorobutylsulfonate anion can be substituted for the respective anion by heating with inorganic (e.g. potassium fluoride ) or organic (e.g. sodium acetate ) salts.
Sulfobutylated β-cyclodextrin
As early as 1949, the reaction of 1,4-butane sultone with the water-insoluble polysaccharide cellulose in sodium hydroxide solution , which leads to a water-soluble product, was reported.
An important application of 1,4- butane sultone derived from this is the derivatization of β-cyclodextrin to sulfobutyl ether-beta-cyclodextrin (SBECD), a water-soluble inclusion compound for the solubilization of poorly water-soluble and for the stabilization of chemically unstable active ingredients.
In the reaction of β-cyclodextrin with 1,4-butane sultone in sodium hydroxide solution at 70 ° C., the sulfobutyl ether is obtained in yields of up to 80% and a degree of substitution (DS) of 6.68.
This increases the solubility of β-cyclodextrin in water at 25 ° C from 18.5 g · l −1 to more than 900 g · l −1 .
Sulfobutylether-beta-cyclodextrin is also used as an inert vehicle for the transport and release of active ingredients ( drug delivery ) in a variety of applications.
Individual evidence
- ↑ a b c d e f g Entry on 1,4-butane sultone in the GESTIS substance database of the IFA , accessed on February 8, 2018(JavaScript required) .
- ↑ Data sheet 1,4-butane sultone from Sigma-Aldrich , accessed on January 16, 2017 ( PDF ).
- ↑ a b c d J.H. Helberger, H. Lantermann: On the knowledge of organic sulfonic acids V. Communication syntheses of 1,4-butane sultone . In: Liebigs Ann. Chem. Band 586 , no. 1 , 1954, p. 158-164 , doi : 10.1002 / jlac.19545860110 .
- ↑ a b c data sheet 1,4-Butanesultone at AlfaAesar, accessed on January 16, 2017 ( PDF )(JavaScript required) .
- ↑ a b c A.O. Snoddy, TL Cairns, WR Brasen,: 4-Hydroxy-1-butanesulfonic acid sultone [1-Butanesulfonic acid, 4-hydroxy-, δ-sultone] In: Organic Syntheses . 37, 1957, p. 55, doi : 10.15227 / orgsyn.037.0055 ; Coll. Vol. 4, 1963, p. 523 ( PDF ).
- ↑ Data sheet 1,4-butane sultone for synthesis (PDF) from Merck , accessed on January 16, 2017.
- ↑ a b H. Ueda, D. Ou, T. Endo, H. Nagase, K. Tomono, T. Nagai: Evaluation of a sulfobutyl ether beta-cyclodextrin as a solubilizing / stabilizing agent for several drugs . In: Drug Dev. Ind. Pharm. Band 24 , no. 9 , 1998, pp. 863-867 , doi : 10.3109 / 03639049809088532 .
- ^ A b V. Wycisk et al .: Responsive Contrast Agents: Synthesis and Characterization of a Tunable Series of pH-Sensitive Near-Infrared Pentamethines . In: ACS Omega . tape 1 , no. 5 , 2016, p. 808-817 , doi : 10.1021 / acsomega.6b00182 .
- ↑ K. Alexander, HV Towles: 4,4'-dichlorobutyl ether In: Organic Syntheses . 30, 1950, p. 27, doi : 10.15227 / orgsyn.030.0027 ; Coll. Vol. 4, 1963, p. 266 ( PDF ).
- ↑ K. Alexander, LE Schniepp: 4,4'-dichlorodibutyl ether and its derivatives from tetrahydrofuran . In: J. Am. Chem. Soc. tape 70 , no. 5 , 1948, pp. 1839–1842 , doi : 10.1021 / ja01185a056 .
- ↑ D. Starr, RM Hixon: Tetramethylene chlorohydrin In: Organic Syntheses . 17, 1937, p. 84, doi : 10.15227 / orgsyn.017.0084 ; Coll. Vol. 2, 1943, p. 571 ( PDF ).
- ↑ Patent EP0222970A1 : Sulfoalkylation process. Registered on July 9, 1986 , published on May 27, 1987 , applicant: Agfa-Gevaert AG, inventor: W. Hünicke, R. Gauglitz.
- ↑ Patent US3146242 : Process for the preparation of sultones. Registered on May 21, 1961 , published on August 25, 1964 , applicant: Henkel & Cie GmbH, inventor: K.-J. Gardenier, H. Kothe.
- ↑ Patent US3117133 : Process for the production of sultones. Registered on October 19, 1960 , published on January 7, 1964 , applicant: Henkel & Cie GmbH, inventor: H. Kothe, K.-J. Gardener.
- ↑ JH Helberger, G. Manecke, HM Fischer: On the knowledge of organic sulfonic acids. II. Mitt .: The sulfochlorination of 1-chlorobutane and other haloalkyls: synthesis of sultones and a sultam . In: Liebigs Ann. Chem. Band 562 , no. 1 , 1949, p. 23-35 , doi : 10.1002 / jlac.19495620104 .
- ↑ L. Fishbein: Potential Industrial Carcinogens and Mutagens, 1st Edition, in Studies in Environmental Science 4 . Elsevier, Amsterdam 1979, ISBN 0-444-41777-X , pp. 124 .
- ↑ D. Wieczorek, A. Dobrowolski, K. Staszak, D. Kwasniewska, P. Dubyk: Synthesis, surface and antimicrobial activity of piperidine-based sulfobetaines . In: J. Surfactants Deterg. tape 20 , no. 1 , 2017, p. 151-158 , doi : 10.1007 / s-11743-016-1906-8 .
- ^ FMA Geilen et al .: Selective and Flexible Transformation of Biomass-Derived Platform Chemicals by a Multifunctional Catalytic System . In: Angew. Chem. Band 49 , no. 32 , 2010, p. 5510–5514 , doi : 10.1002 / anie.201002060 .
- ↑ Patent US3870750 : Process for the production of aminomethane-diphosphonic acid and its salts. Applied on April 26, 1972 , published on March 11, 1975 , Applicant: Henkel & Cie GmbH, Inventors: K. Wollmann, W. Plöger, K.-H. Wopms.
- ↑ Patent US4250107 : N- (sulfoalkane) amino alkane phosphonic acids and their water-soluble salts. Registered on November 6, 1978 , published on February 10, 1981 , applicant: Benckiser-Knapsack GmbH, inventor: K. Sommer, G. Schoebel.
- ↑ AR Hajipour, Y. Ghayeb, N. Sheikhan, AE Ruoho: Brønsted acidic ionic liquid as an efficient and reusable catalyst for one-pot synthesis of 1-amidoalkyl 2-naphthols under solvent-free conditions . In: Tetrahedron Lett. tape 50 , 2009, p. 5649-5651 , doi : 10.1016 / j.tetlet.2009.07.116 .
- ↑ N. Paape, W. Wie, A. Bösmann, C. Kolbeck, F. Maier, H.-P. Steinrück, P. Wasserscheid, PS Schulz: Chloroalkylsulfonate ionic liquids by ring opening of sultones with organic chloride salts . In: Chem. Commun. 2008, p. 3867-3869 , doi : 10.1039 / B805444D .
- ↑ Patent WO2009152902A2 : Ionic liquids. Applied on May 12, 2009 , published on December 23, 2009 , Applicant: Merck Patent GmbH, Inventors: P. Wasserscheid, N. Paape, A. Boesmann, P. Schulz.
- ↑ JH Helberger, G. Manecke, R. Heyden: On the knowledge of organic sulfonic acids III. Communication: The alkylation reactions of the sultones . In: Liebigs Ann. Chem. Band 565 , no. 1 , 1949, p. 22-35 , doi : 10.1002 / jlac.19495650104 .
- ↑ The United States Pharmacopeia 38th ed., National Formulary 33th: Betadex Sulfobutyl Ether Sodium . Ed .: United States Pharmacopeial Convention. Rockville, MD 2015, ISBN 978-1-936424-34-4 , pp. 6546-6548 .
- ^ T. Loftsson, D. Duchene: Cyclodextrins and their pharmaceutical applications . In: Int. J. Pharm. Volume 329 , no. 1–2 , 2007, pp. 1–11 , doi : 10.1016 / j.ijpharm.2006.10.044 .
- ↑ a b S. Klein, T. Zöller: Cyclodextrins: Molecular sugar bags for drugs . In: Pharm. Ztg. Volume 26 , 2008 ( online ).
- ↑ D.-Y. Ma, Y.-M. Zhang, J.-N. Xu: The synthesis and process optimization of sulfo butyl ether β-cyclodextrin derivatives . In: Tetrahedron . tape 72 , no. 22 , 2016, p. 3105–3112 , doi : 10.1016 / j.tet.2016.04.039 .
- ↑ R. Challa, A. Ahuya, J. Ali, RK Khar: Cyclodextrins in drug delivery: an updated review . In: AAPS Pharm. Sci. Tech. tape 6 , no. 2 , 2005, p. E329-E357 , doi : 10.1208 / pt060243 .