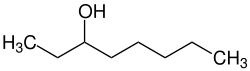
3-octanol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| Simplified structural formula without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 3-octanol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 18 O | ||||||||||||||||||
| Brief description |
colorless liquid with an aromatic odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 130.23 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.82 g cm −3 |
||||||||||||||||||
| Melting point |
−45 ° C |
||||||||||||||||||
| boiling point |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.426 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
3-Octanol is a chemical compound from the group of alcohols , more precisely the chiral alcohols.
Occurrence
3-Octanol occurs naturally in spearmint oil , oat groats , basil , allspice leaves , and truffles . The compound has been detected (free and esterified) in a variety of mints , lavender , the essential oils of field mint and spearmint , in apples, bananas, cranberries , grapes, papaya , strawberries , sour cherries , lemons , blackberries , pork, peas , potatoes , Ginger , thyme , fish, fried beef, cognac , rum , wine , coffee , tea , oats , soybeans , mushrooms, marjoram , seaweed , buckwheat , lemon balm , truffles, passion fruit and pepino fruit (Solanum muricatum).
It serves as an alarm pheromone for some ants . The mandible glands of the ant species Myrmica contain 3-octanol, 90% of it in the form of the ( R ) - enantiomer , among other substances . The species M. rubra and scabrinodis react specifically to this ( R ) -isomer.
Extraction and presentation
3-Octanol can be produced in racemic form by reducing 3-octanone with sodium in an ether solution.
properties
3-Octanol is a flammable, hardly inflammable, colorless liquid with an aromatic odor that is practically insoluble in water.
use
3-Octanol is used as a flavoring agent (e.g. for lavender and mushroom smells).
safety instructions
The vapors of 3-octanol can form an explosive mixture with air ( flash point 68 ° C).
Individual evidence
- ↑ Entry on 3-OCTANOL in the CosIng database of the EU Commission, accessed on January 21, 2020.
- ↑ a b c d e f g h i j k Entry on 3-octanol in the GESTIS substance database of the IFA , accessed on January 21, 2020(JavaScript required) .
- ↑ a b Data sheet (S) - (+) - 3-octanol, 97% from Sigma-Aldrich , accessed on April 23, 2017 ( PDF ).
- ↑ a b c d George A. Burdock: Fenaroli's Handbook of Flavor Ingredients, Sixth Edition . CRC Press, 2016, ISBN 978-1-4200-9086-4 , pp. 1526 ( limited preview in Google Book search).
- ↑ a b Data sheet 3-Octanol, natural, ≥97%, FCC, FG from Sigma-Aldrich , accessed on April 23, 2017 ( PDF ).
- ↑ John L. Capinera: Encyclopedia of Entomology . Springer Science & Business Media, 2008, ISBN 978-1-4020-6242-1 , p. 92 ( limited preview in Google Book search).
- ↑ MC. CAMMAERTS, K. MORI: Behavioral activity of pure chiral 3-octanol for the ants Myrmica scabrinodis Nyl. and Myrmica rubra L. In: Physiological Entomology. 12, 1987, p. 381, doi: 10.1111 / j.1365-3032.1987.tb00764.x .
- ^ Wiley-VCH: Ullmann's Food and Feed, 3 Volume Set . John Wiley & Sons, 2017, ISBN 978-3-527-33990-7 , pp. 1059 ( limited preview in Google Book search).

