Avobenzone
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
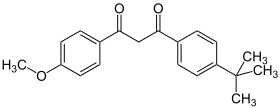
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Avobenzone | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 20 H 22 O 3 | |||||||||||||||||||||
| Brief description |
whitish powder or slightly yellow solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 310.39 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.037-1.041 g · cm -3 |
|||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Avobenzon (German INN ; mostly the English INN Avobenzone is also used in German ) is a substituted 1,3- diketone .
Nm with its maximum absorption at 357 is avobenzone an effective UV-A - sunscreens , which effectively absorbs UV radiation and the formation of free radicals effectively suppressed. Avobenzone is used worldwide and is the only organic UV absorber currently approved for use in sunscreens in the USA with absorption in the longer-wave UVA range (315-380 nm).
Manufacturing
In a Claisen condensation , methyl 4- tert- butylbenzoate (from 4- tert- butylbenzoic acid by esterification with methanol ) reacts with 4-methoxyacetophenone in toluene and in the presence of sodium amide to give 4- tert- butyl-4'-methoxydibenzoylmethane in 71% yield :
According to a more recent patent application, yields of up to 95% are achieved with the same starting materials in toluene in the presence of potassium methoxide .
properties
Pure avobenzone is a whitish to yellowish crystalline powder with a faint odor that dissolves in isopropanol , decyl oleate , capric acid / caprylic acid triglycerides and other oils.
As an enolate, avobenzone forms with heavy metal ions, such as B. Fe 3+ colored complexes, to suppress chelating agents such. B. EDTA can be added. Stearates , aluminum , magnesium and zinc salts can lead to poorly soluble precipitates.
It is subject to a keto-enol tautomerism and is predominantly available as enol :
Avobenzone shows different photostabilities depending on the polarity of the solvents used. Avobenzone is more photostable in polar media that promote the formation of intermolecular hydrogen bonds between avobenzone and the solvent. In non-polar media, it photoisomerizes into a triplet excited state of the diketo form, which can transfer its energy to other biomolecules and damage them. The excited keto form easily photolyzes with cleavage of bonds to form phototoxic and photoallergic degradation products.
Due to the photoreactivity, the UV absorption and thus the light protection effect of avobenzone decrease. Among the common lipophilic pharmaceutical bases, avobenzone is the most stable when exposed to UV radiation in mineral oil and isopropyl myristate .
The light-induced degradation of avobenzone can be reduced by adding photostabilizers, including photostable organic UV filters such. B. Octocrilen , which absorb the energy of the excited Avonbenzone, by antioxidants such. B. vitamin E , vitamin C or ubiquinone or with cyclodextrins significantly reduce.
Applications
Avobenzone is the most important UV-A filter in sunscreens on the American market. UV filters are in the US as drug (Engl. Active Pharmaceutical Ingredients , API), are therefore subject sunscreen as OTC drugs (Engl. OTC drugs regulated by the Food and Drug Administration () Food and Drug Administration , FDA) and the guidelines of Good Manufacturing Practice (cGMP). Since avobenzone was approved by the FDA in 1988, no new UV-A absorber has been launched in the USA, which is why only avobenzone is permitted in a maximum concentration of 3% (Europe 5%, up to 10% in Japan) . To increase the sun protection factor (Engl. Sun protection factor , SPF) must therefore photostabilizers and increasingly so-called SPF boosters are used in formulations.
Mixtures with the inorganic UV absorbers titanium dioxide and zinc oxide also increase the sun protection factor and the UV stability of avobenzone, but the surface of the TiO 2 particles must be coated because of their high photocatalytic activity.
Because they suppress the formation of free radicals in cosmetics, UV absorbers such as avobenzone are also touted as effective against skin aging ( anti-aging ), against skin wrinkles ( anti-wrinkle ) and as anti-inflammatory ( anti-inflammatory ).
Avobenzone forms stable crystalline complexes with boron trifluoride , which fluoresce under UV radiation, and undergo a (reversible) color change under mechanical stress.
Risk assessment
Avobenzon was included in the EU's ongoing action plan ( CoRAP ) in 2015 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the inclusion of avobenzone were concerns about consumer use , environmental exposure , high (aggregated) tonnage and widespread use, as well as the dangers arising from a possible assignment to the group of PBT / vPvB substances. The re-evaluation has been running since 2015 and is carried out by Germany . In order to be able to reach a final assessment, further information was requested.
Trade names
- Neo Heliopan 357, Parsol 1789, Eusolex 9020
Individual evidence
- ↑ Entry on BUTYL METHOXYDIBENZOYLMETHANE in the CosIng database of the EU Commission, accessed on December 28, 2019.
- ↑ a b c data sheet Avobenzone at Sigma-Aldrich , accessed on May 18, 2017 ( PDF ).
- ↑ a b c data sheet Eusolex® 9020 (PDF) from Merck , accessed on July 25, 2015.
- ↑ a b c Entry on 1- (4- (1,1-Dimethylethyl) phenyl) -3- (4-methoxyphenyl) propane-1,3-dione, avobenzone in the GESTIS substance database of the IFA , accessed on February 1 2016(JavaScript required) .
- ↑ a b c Kuhnert-Brandstätter, M .; Völlenklee, R .: Contribution to the polymorphism of medicinal substances, Part 4: Oxamniquin, Resorantel, Spiperon, Suloctidil, Ticlopidinhydrochlorid, Parsol 1789 and Testosteroncyptonat in Sci. Pharm. 55 (1987) 27-39.
- ^ A b LyondellBasell: Application Data, Solubility Screen, Ultraviolet Light Absorbers .
- ↑ Decision of the Commission of February 9, 2006 amending Commission Decision 96/335 / EC establishing a list and a common nomenclature of the ingredients of cosmetic products .
- ↑ G. Vielhaber, S. Grether-Beck, O. Koch, W. Johncock, J. Krutmann: Sunscreens with an absorption maximum of ≥360 nm provide optimal protection against UVA1-induced expression of matrix metalloproteinase-1, interleukin-1, and interleukin-6 in human dermal fibroblasts . In: Photochem. Photobiol. Sci. tape 5 , 2006, p. 275-282 , doi : 10.1039 / B516702G .
- ^ A b D. G. Beasley, TA Meyer: Characterization of the UVA protection provided by avobenzone, zinc oxide, and titanium dioxide in broad-spectrum sunscreen products . In: American Journal of Clinical Dermatology . tape 11 , no. 6 , 2010, p. 413-421 , doi : 10.2165 / 11537050-000000000-00000 (English).
- ↑ Patent US4387089 : 4- (1,1-Dimethylethyl) -4'-methoxydibenzoylmethane. Applied on May 18, 1981 , published June 7, 1983 , applicant: Givaudan Corp., inventor: K.-F. De Polo.
- ↑ Patent WO2012084770A1 : Process for the manufacture of dibenzoylmethane derivatives. Registered on December 19, 2011 , published on June 28, 2012 , applicant: DSM IP Assets BV, inventor: C. Wehrli.
- ↑ a b Making Cosmetics®, Avobenzone. In: makingcosmetics.com. Retrieved July 29, 2015 .
- ↑ a b G. J. Mturi, BS Martincigh: Photostability of the sunscreening agent 4-tert-butyl-4-methoxydibenzoylmethane (avobenzone) in solvents of different polarity and proticity . In: J. Photochem. Photobiol .: Chemistry . tape 200 , 2008, p. 410-420 , doi : 10.1016 / j.jphotochem.2008.09.007 (English).
- ↑ C. Paris, V. Lhiaubet-Vallet, O. Jiménez, C. Trullas, M. Angel Miranda: A Blocked Diketo Form of Avobenzone: Photostability, Photosensitizing Properties and Triplet Quenching by a Triazine-derived UVB filter . In: Photochem. Photobiol. tape 85 , no. 1 , 2009, p. 178-184 , doi : 10.1111 / j.1751-1097.2008.00414.x (English).
- ^ JJ Vallejo, M. Mesa, C. Gallardo: Evaluation of the avobenzone photostability in solvents used in cosmetic formulations
- ↑ S. Afonso et al: Photodegradation of avobenzone: Stabilization effect of antioxidants . In: J. Photoche. Photobiol .: Biology . tape 140 , 2014, p. 36-40 , doi : 10.1016 / j.photobiol.2014.07.004 (English).
- ↑ J. Yang, CD Wiley, DA Godwin, LA Felton: Influence of hydroxypropyl-β-cyclodextrin on transdermal penetration and photostability of avobenzone . In: Eur. J. Pharm. Biopharm. tape 69 , no. 2 , 2008, p. 605-612 , doi : 10.1016 / ejpb.2007.12.015 (English).
- ↑ MS Reisch: After More Than A Decade, FDA Still Won't Allow New Sunscreens . In: Chemical & Engineering News . tape 93 , no. 20 , 2015, p. 10-15 ( acs.org ).
- ↑ NA Shaath: SPF Boosters & Photo Stability of ultraviolet filter. HAPPI, October 2007.
- ↑ U. Nguyen, D. Schlossman: Stability Study of Avobenzone with Inorganic Sunscreens. ( Memento of February 7, 2012 on the Internet Archive ) Kobo Products, Inc., (PDF file).
- ↑ G. Zhang, J. Lu, M. Sabat, CL Fraser: Polymorphism and Reversible Mechanochromic Luminescence for Solid-State Difluoroboron Avobenzone . In: J. Am. Chem. Soc. tape 132 , no. 7 , 2010, p. 2160–2162 , doi : 10.1021 / ja9097719 (English).
- ^ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): 1- [4- (1,1-dimethylethyl) phenyl] -3- (4-methoxyphenyl) propane-1,3-dione , accessed on March 26th 2019.


