Prednisolone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
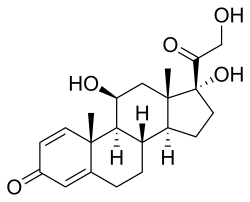
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Prednisolone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 21 H 28 O 5 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code |
A07 EA01 , C05 AA04 , D07 AA03 , D07 XA02 , H02 AB06 , R01 AD02 , S01 BA04 , S01 CB02 , S02 BA03 , S03 BA02 |
||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 360.44 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
239 ° C |
||||||||||||||||||
| solubility |
very bad in water (223 mg l −1 at 25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Prednisolone (1,2-dehydro cortisol ; formerly also called metacortandralone or delta hydrocortisone ) is a synthetic glucocorticoid . Prednisolone is an active metabolite of prednisone .
Prednisolone has been marketed by Merck in Germany as "Solu-Decortin H" since 1957 .
Physical Properties
Prednisolone is a solid crystalline substance that occurs in two polymorphic forms and one hydrate form. The commercial product melts at 239 ° C with a heat of fusion of 33.1 kJ mol −1 . The second polymorph shows a melting point at 243 ° C with a heat of fusion of 23.7 kJ mol −1 . Both crystal forms are thus enantiotropic to one another, the lower melting polymorph being the thermodynamically more stable form at room temperature. The hydrate form has a 1.5 stoichiometry and can be crystallized from methanol-water mixtures. The water of hydration is released from 30 ° C with the formation of the higher melting crystal form. All three crystal forms show a different solubility.
effect
Prednisolone has a pronounced immunosuppressive and also anti-inflammatory, anti-allergic effect. It is the active ingredient of choice for systemic anti-inflammatory and immunosuppressive effects.
application areas
The active ingredient is used both in free form and as prednisolone acetate wherever acute inflammatory reactions have to be suppressed. These include: shock / anaphylactic shock , severe forms of allergic reactions , for example after wasp stings, brain edema, meningitis , after neurosurgical operations , transplants (if there is a risk of organ rejection), inhalation of toxic substances such as B. chlorine gas , pseudo croup , severe infectious diseases or acute worsening of chronic obstructive pulmonary disease (COPD) and severe asthma level 4 , rheumatoid diseases, inflammatory eye diseases, chronic inflammatory bowel diseases, multiple sclerosis and autoimmune diseases, skin diseases (especially neurodermatitis ) even if not reliably and conclusively proven - in the treatment of a sudden hearing loss or even just acute idiopathic tinnitus (duration up to three months).
In the case of pericardial inflammation caused by immune diseases, active substances based on glucocorticoids can be administered.
Another area of application of prednisolone is the preventive treatment of cluster headache .
Side effects
Prednisolone has numerous side effects that can also be dangerous (see also Glucocorticoids # Side Effects ). The immunosuppressive effect results in the affected patient being more susceptible to infection. If taken for a long time, a catabolic effect leads to damage to the bone structure ( osteopenia or osteoporosis ) and an iatrogenic Cushing's syndrome (caused by medical therapy) . Diabetes mellitus can also develop .
When stopping long-term therapy with prednisolone or other steroids, it may be necessary to slowly reduce the daily dose, which is known as "tapering". Failure to do this can lead to acute steroid withdrawal syndrome , which is similar to adrenal insufficiency . Blood levels of spontaneous morning cortisol levels (which should be above 500 nmol / l) immediately after the end of prednisolone therapy can help prevent this.
Equivalent doses
The relative anti-inflammatory equivalent dose of other preparations, which must be considered if prednisolone is to replace the intake of another glucocorticoid, can be read from the following equation:
5 mg prednisolone = 5 mg prednisone = 0.7 mg dexamethasone = 4 mg triamcinolone = 4 mg methylprednisolone = 20 mg hydrocortisone = 25 mg cortisone .
Trade names
- Monopreparations : Aprednislon (A), Decortin H (D), Dermosolon (D), Dontisolon (D), Hefasolon (D), Hexacorton (CH), Infectocortikrupp (D), Inflanefran (D), Klismacort (D), Kuehlprednon ( A), Linola H (D), Lygal tincture (D), Prednisolon (D), Prednisolut (D), Premandol (CH), Solu-Decortin H (D), Solu-Dacortin (A), Spiricort (CH), Ultracortenol (D, A, CH), Prednison 5 léčiva (CZ, SK), numerous generics (D, A, CH)
- Combination preparations: Alpicort (D, A, CH), Aquapred (D), Bismolan-H Corti (D), Blephamide (D, CH), Imacort (CH), Imazol comp (D), Inflanegent (D), Leioderm P ( D), Linoladiol H (D), Locaseptil (CH), Mycinopred (CH), Oxytetracycline-Prednisolon (D), Scheriproct (A, CH)
Individual evidence
- ↑ a b c d e f M. D. Veiga, R. Cadorniga: Thermal Study of Prednisolone Polymorphs , in: Thermochim. Acta , 1985 , 96 , pp. 111-115, doi : 10.1016 / 0040-6031 (85) 80012-5 .
- ↑ a b Entry on prednisolone in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Datasheet Prednisolone from Sigma-Aldrich , accessed on April 22, 2011 ( PDF ).
- ↑ Horst Finger: The influence of Avil and Solu-Decortin H on experimental “allergic” encephalomyelitis in guinea pigs and rabbits. Research in Experimental Medicine , Springer, Berlin / Heidelberg 1961 , 135 (3), pp. 276-280, doi : 10.1007 / BF02045076 .
- ↑ MD Veiga, YR Cadorniga: Estudio de coeficientes de solubilidad y velocidad de disolución de diversos polimorfos de la prednisolona. in: Cien. Ind. Farm. , 1988 , 7 , pp. 201-205.
- ↑ Pharmacology / HP Rang et al. - Churchill Livingstone, Edinburgh 2003, p. 412.
- ↑ Guideline Tinnitus of the German Society for Ear, Nose and Throat Medicine, Head and Neck Surgery . In: AWMF online (as of February 1, 2010, in revision).
- ^ S1 guideline for cluster headaches and trigemino-autonomic headaches of the German Society for Neurology (DGN). In: AWMF online (as of 2015).
- ↑ B. Kreutzkamp, in Medical Tribune 49, July 11, 2014, p. 4.