Carfentrazone-ethyl
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
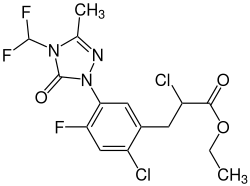
|
|||||||||||||||||||
| Simplified structural formula without stereochemistry 1: 1 mixture of the ( R ) - and the ( S ) -form |
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Carfentrazone-ethyl | ||||||||||||||||||
| other names |
Ethyl ( RS ) -2-chloro-3- (2-chloro-4-fluoro-5- (4-difluoromethyl-4,5-dihydro-3-methyl-5-oxo-1 H -1,2,4 -triazol-1-yl) phenyl) propionate |
||||||||||||||||||
| Molecular formula | C 15 H 14 Cl 2 F 3 N 3 O 3 | ||||||||||||||||||
| Brief description |
colorless to yellow, viscous liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 412.19 g · mol -1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.46 g · cm -3 |
||||||||||||||||||
| Melting point |
−22.1 ° C |
||||||||||||||||||
| boiling point |
350 ° C |
||||||||||||||||||
| Vapor pressure |
7.2 10 −6 Pa (20 ° C) |
||||||||||||||||||
| solubility |
practically insoluble in water (29.3 mg l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data |
|
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Carfentrazone-ethyl is a herbicide from the triazolinones group of active ingredients . It was launched by FMC in 1993 .
properties
Carfentrazone-ethyl is a colorless to yellow, viscous liquid with a slightly petroleum-like odor. It is almost insoluble in water, but easily soluble in organic solvents. With a half-life of one day, it is not persistent in the soil. Carfentrazone-ethyl is light-sensitive and susceptible to hydrolysis . It is stable under acidic to neutral conditions, but degrades rapidly in alkaline conditions . The molecule has a stereocenter . So there are two possible stereoisomers . The herbicide carfentrazone-ethyl is used as a racemate .
Mode of action and use
Carfentrazone-ethyl is a post-emergence herbicide and is effective against broad-leaved weeds and some mustard plants . It is mainly used in the cultivation of grain , maize , soy , potatoes and wine as well as to control weeds on sports facilities and golf courses. It works in direct contact with the target plants. It is activated by sunlight and inhibits the function of the enzyme protoporphyrinogen oxidase , which is responsible for chlorophyll synthesis. In the process, phytotoxic intermediate products are formed which accumulate in the cell and destroy the weed's cell membrane . As a result, these plants die within a few days.
toxicology
In animal experiments , it was found that the inhibition of protoporphyrinogen oxidase in mammals can impair the biosynthesis of the blood pigment heme . This can change the blood count and increase the concentration of porphyrins in the urine . Nevertheless, carfentrazone-ethyl is classified as having little acute toxicity in the case of oral, inhalative or dermal absorption. It is also not irritating to the skin or the eyes and does not sensitize the skin. No carcinogenic or genotoxic effects could be determined. The European Food Safety Authority (EFSA) set the permitted daily dose at 0.03 mg / kg body weight.
Admission
Carfentrazone-ethyl was approved by the European Union on July 11, 2003 . Plant protection products containing the active ingredient are available in Germany, Austria and Switzerland. In part, it is combined with metsulfuron-methyl .
Individual evidence
- ↑ a b c d e f g h i j k l Entry on Carfentrazone-ethyl in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on February 21, 2020.
- ↑ Entry on Carfentrazone-ethyl in the GESTIS substance database of the IFA , accessed on February 20, 2020(JavaScript required) .
- ↑ Entry on Carfentrazone-ethyl in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 21, 2020. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Safety data sheet according to Regulation (EC) No. 1907/2006 (REACH). (PDF) HPC Standards GmbH, November 13, 2017, accessed on March 11, 2020 .
- ↑ James A. Kent: Kent and Riegel's Handbook of Industrial Chemistry and Biotechnology . Springer Science & Business Media, 2010, ISBN 978-0-387-27843-8 ( limited preview in Google book search).
- ↑ a b c Paranjape, Kalyani .: The pesticide encyclopedia . CABI, Wallingford, Oxfordshire UK 2014, ISBN 978-1-78064-014-3 ( limited preview in Google Book Search).
- ↑ a b Peer review of the pesticide risk assessment of the active substance carfentrazone-ethyl . In: EFSA Journal . tape 14 , no. 8 , 2016, ISSN 1831-4732 , doi : 10.2903 / j.efsa.2016.4569 .
- ↑ Chauhan, Bhagirath Singh ,, Mahajan, Gulshan,: Recent advances in weed management . New York, NY, ISBN 978-1-4939-1019-9 ( limited preview in Google Book Search).
- ↑ Directive 2003/68 / EG (PDF; 130 kB)
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on carfentrazone-ethyl in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 25, 2020.
