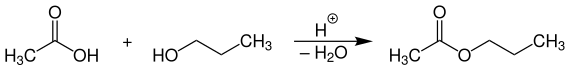Acetic acid n- propyl ester
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Acetic acid n- propyl ester | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 5 H 10 O 2 | |||||||||||||||||||||
| Brief description |
volatile, colorless liquid with a fruity odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 102.13 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
0.89 g cm −3 (20 ° C) |
|||||||||||||||||||||
| Melting point |
−95 ° C |
|||||||||||||||||||||
| boiling point |
102 ° C |
|||||||||||||||||||||
| Vapor pressure |
|
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| Refractive index |
1.3844 |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
DFG / Switzerland: 100 ml m −3 or 420 mg m −3 |
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Acetic acid n- propyl ester (according to IUPAC nomenclature: n-propyl ethanoate , also known as n-propyl acetate ) is an organic-chemical compound from the group of carboxylic acid esters . Together with the isomeric compound isopropyl acetate , it forms the group of propyl acetates .
Extraction and presentation
The industrial production of n -propyl acetate takes place as far as possible by direct esterification of acetic acid with n -propanol at temperatures of 90-120 ° C on strongly acidic cation exchangers as a catalyst .
The reaction is carried out in a tubular fixed-bed reactor and the water formed is continuously removed from the reaction mixture (shifting the equilibrium to the product side).
In the laboratory or on a smaller scale, strong mineral acids such as sulfuric or hydrochloric acid , and often p -toluenesulfonic acid , are also used as catalysts.
properties
Physical Properties
Acetic acid n -propyl ester is a colorless liquid that boils at 101.5 ° C under normal pressure . The heat of vaporization at the boiling point is 33.92 kJ mol −1 . According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 4.14386, B = 1283.861 and C = −64.378 in the temperature range from 312.22 to 374.03 K. It has a specific heat capacity at 25 ° C of 196.07 J · K −1 · mol −1 or 1.92 J · K −1 · g −1 , one Surface tension of 24.28 mN / m, a standard enthalpy of vaporization of 39.77 kJ / mol, a dielectric constant of 6.002 and a viscosity of 0.58 mPa · s (in each case at 20 ° C).
Safety-related parameters
Acetic acid n -propyl ester forms highly flammable vapor-air mixtures. The compound has a flash point of 10 ° C. The explosion range is between 1.7% by volume (70 g / m 3 ) as the lower explosion limit (LEL) and 8.0% by volume (340 g / m 3 ) as the upper explosion limit (UEL).) The maximum explosion pressure is 8.6 bar. The limit gap width was determined to be 1.04 mm. This results in an assignment to explosion group IIA. The ignition temperature is 455 ° C. The substance therefore falls into temperature class T1.
use
Acetic acid n- propyl ester is used as a solvent in the paint, printing inks and chemical-technical industries (e.g. for cosmetics and adhesives). It has good dissolving power for numerous natural and synthetic resins (e.g. cellulose nitrate ) and is easily biodegradable.
Toxicology / Risk Assessment
The vapors of acetic acid n- propyl ester have a narcotic effect on humans.
Acetic acid n propyl was in 2015 by the EU under the Regulation (EC) no. 1907/2006 (REACH) under the substance evaluation in the ongoing Community Action Plan ( CoRAP added). The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The causes of the uptake of n- propyl acetate were concerns about consumer use , exposure of workers , high (aggregated) tonnage, other hazard-related concerns and widespread use, as well as the potential risk from reproductive properties. The re-evaluation is expected to be carried out by Ireland from 2021 .
Web links
- Entry on Propyl acetate in the Spectral Database for Organic Compounds (SDBS) of the National Institute of Advanced Industrial Science and Technology (AIST)
Individual evidence
- ↑ Entry on PROPYL ACETATE in the CosIng database of the EU Commission, accessed on June 29, 2020.
- ↑ a b c d e f g h i j k l m n o p q r Entry on propyl acetate in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b BASF: n-Propyl Acetate , accessed on February 2, 2020
- ^ Wilhelm Riemenschneider, Hermann M. Bolt: Esters, Organic. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley ‐ VCH Verlag GmbH & Co. KGaA., April 30, 2005, p. 247, doi : 10.1002 / 14356007.a09_565.pub2 .
- ↑ Entry on Propyl acetate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 109-60-4 or n-propyl acetate ), accessed on November 2, 2015.
- ↑ a b Patent EP0066059B1 : Process for the esterification of acetic acid with C2 to C5 alcohols. Published on November 28, 1984 , applicant: Chemische Werke Hüls AG, inventors: Helmut Alfs, Werner Böxkes, Erwin Vangermain.
- ↑ Entry on propyl acetate. In: Römpp Online . Georg Thieme Verlag, accessed on November 21, 2018.
- ↑ Svoboda, V .; Vesely, F .; Holub, R .; Pick, J .: Heats of vaporization of alkyl acetates and propionates in Collect. Czech. Chem. Commun. 42 (1977) 943-951.
- ↑ a b Majer, V .; Svoboda, V .: Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation , Blackwell Scientific Publications, Oxford, 1985, 300.
- ↑ Polák, J .; Mertl, I .: Saturated vapor pressure of methyl acetate, ethyl acetate, n-propyl acetate, methyl propionate, and ethyl propionate in Collect. Czech. Chem. Commun. 30 (1965) 3526-3528, doi : 10.1135 / cccc19653526 .
- ↑ Jimenez, E .; Romani, L .; Paz Andrade, MI; Roux-Desgranges, G .; Grolier, J.-PE: Molar excess heat capacities and volumes for mixtures of alkanoates with cyclohexane at 25 ° C in J. Solution Chem. 15 (1986) 879-890, doi : 10.1007 / BF00646029 .
- ↑ a b c E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): propyl acetate , accessed on March 26, 2019.