Hydroxyethyl methacrylate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
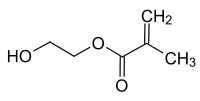
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Hydroxyethyl methacrylate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 10 O 3 | |||||||||||||||
| Brief description |
colorless liquid with a fruity odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 130.14 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.07 g cm −3 |
|||||||||||||||
| Melting point |
−12 ° C |
|||||||||||||||
| boiling point |
250 ° C |
|||||||||||||||
| Vapor pressure |
1.3 h Pa (20 ° C) |
|||||||||||||||
| solubility |
miscible with water |
|||||||||||||||
| Refractive index |
1.453 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2-Hydroxyethyl methacrylate , or HEMA for short , is a chemical compound from the group of substituted carboxylic acid esters and alcohols .
Extraction and presentation
2-Hydroxyethyl methacrylate is produced by reacting methacrylic acid with ethylene oxide in the presence of hydroquinone . In 1999 around 42,000 t were produced worldwide. This means that 2-hydroxyethyl methacrylate is one of the chemical substances that are produced in large quantities (" High Production Volume Chemical ", HPVC) and for which the Organization for Economic Cooperation and Development (OECD) collects data on possible dangers (" Screening Information Dataset “, SIDS) was made.
properties
2-Hydroxyethyl methacrylate is a difficult to ignite, colorless liquid with a fruity odor. Your aqueous solution is acidic. The heat of polymerization is −50 kJ mol −1 or −384 kJ kg −1 . The distribution coefficient log K ow (medium: octanol-water) is 0.42.
The vapors of 2-hydroxyethyl methacrylate can form an explosive mixture with air (flash point 101 ° C), from 375 ° C self-ignition occurs.
Spectroscopy
NMR spectroscopy
The 1 H-NMR spectrum (measured in CDCl 3 ) shows five signals: δ: 1.90 (t, 3 H, Me, J = 1.3 Hz), 3.80 (t, 2 H, CH 2 O , J = 6.5 Hz), 4.25 (t, 2H, CH 2 O, J = 6.5 Hz), 5.10 (t, 1H, = CH 2 , J = 1.3 Hz), 6.10 (s, 1H, = CH 2 ) ppm. If the measurement is made in DMSO-d6 , the proton of the alcohol group is also visible and six signals result.
IR spectroscopy
Characteristic IR bands of HEMA (measured as a film between KBr disks) include the broad OH stretching vibration of the associates formed by hydrogen bonds around 3500 cm −1 , the strong asymmetrical CH stretching shrinkage around 2970 cm −1 , the strong band at about 1720 cm −1 , which is associated with the C = O stretching vibration (ester) and which is followed by the narrow, medium-strong C = C stretching vibration of the vinylidene group at around 1640 cm −1 . Overall, all the bands in the IR spectrum can be easily assigned to the functional groups.
use
2-Hydroxyethyl methacrylate is used as a reactive diluent in radical radiation curing and as a comonomer for the production of acrylic resins . It is also for the production of acrylic - polymer (as polyHEMA or as a copolymer with methacrylic acid, styrene , methyl methacrylate , butyl acrylate and other), as dental filling plastics , contact lenses , artificial fingernails (made of UV light -gehärteten acrylates), in the platemaking (Printing industry) and in acrylic resin varnishes. Wicherle and Lim were the first to show that a hydrogel based on hydroxyethyl methacrylate could be suitable as a biocompatible material. In 1953 Otto Wichterle and the chemist Drahoslav Lím patented a manufacturing process.
toxicity
Toxicokinetics
In mammals, HEMA is rapidly hydrolyzed to ethylene glycol (EG) and methacrylic acid (MAA) by esterases . The last metabolites are CO 2 , which is exhaled and oxalic acid, which is excreted in the urine. The estimated half-life of HEMA in mammalian metabolism is on the order of a few minutes.
Inhalative toxicity
In view of the low vapor pressure, the possible inhalation of HEMA is not a relevant route of exposure; potentially toxic concentrations cannot be achieved in this way.
Oral toxicity
HEMA shows low acute oral toxicity in studies on rats. Available studies consistently give an oral LD 50 for rats of> 5,000 mg / kg body weight.
The oral NOAEL for rats is given as 30 mg / kg body weight.
Dermal toxicity
In the available studies , the dermal LD 50 for rabbits is consistently given as> 3,000 mg / kg body weight.
The risk of absorption of toxic doses in humans appears to be low, also due to the rapid metabolism of HEMA.
Awareness
HEMA has been classified as sensitizing in animal experiments and in humans.
Genotoxicity and Mutagenicity
Both positive and negative results in in vitro and in vivo mutagenicity / genotoxicity tests are available for HEMA . Overall, it is classified as not mutagenic according to internationally recognized criteria.
Risk assessment
Hydroxyethyl methacrylate was included in the EU's ongoing action plan ( CoRAP ) in 2013 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The causes for the uptake of hydroxyethyl methacrylate were concerns about consumer use , high (aggregated) tonnage, high risk characterization ratio (RCR) and widespread use as well as the dangers arising from a possible assignment to the group of CMR substances and the suspected dangers sensitizing properties. The re-evaluation has been running since 2014 and is carried out by France .
Individual evidence
- ↑ a b c d e f g h i j k l m n o p Entry on 2-hydroxyethyl methacrylate in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b Data sheet 2-Hydroxyethyl methacrylate from Sigma-Aldrich , accessed on November 12, 2018 ( PDF ).
- ↑ Entry on 2-hydroxyethyl methacrylate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ Textbook of organic chemistry, Beyer / Walter
- ↑ Entry on (2-hydroxyethyl) methacrylate. In: Römpp Online . Georg Thieme Verlag, accessed on November 3, 2014.
- ↑ OECD : Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for 2-Hydroxyethyl methacrylate , accessed on October 3, 2014.
- ↑ Brandrup, J .; Immergut, EH; Grulke, EA; Abe, A .; Bloch, DR: Polymer Handbook , 4th Edition, Wiley-VCH 2003, ISBN 978-0-471-47936-9 , p. II / 369.
- ↑ a b Seda A. Torosyan, Yulia N. Biglova, Vladimir V. Mikheev, Zarina T. Khalitova, Fanuza A. Gimalova: Synthesis of fullerene-containing methacrylates . In: Mendeleev Communications . tape 22 , no. 4 , 2012, p. 199–200 , doi : 10.1016 / j.mencom.2012.06.009 .
- ↑ Prantik Mondal, Prasanta Kumar Behera, Nikhil K. Singha: A healable thermo-reversible functional polymer prepared via RAFT polymerization and ultrafast 'click' chemistry using a triazolinedione derivative . In: Chemical Communications . tape 53 , no. 62 , 2017, p. 8715-8718 , doi : 10.1039 / C7CC02980B .
- ↑ a b Entry on 2-hydroxyethyl methacrylate in the Spectral Database for Organic Compounds (SDBS) of the National Institute of Advanced Industrial Science and Technology (AIST), accessed on November 17, 2019.
- ↑ Information on contact allergens
- ↑ Jörg Zimmermann: Polyol and azlactone macromonomers for network systems, new materials and biomedical applications. Dissertation, University of Freiburg, 2001 DNB 96377381x / 34
- ↑ a b c d e f g Registration dossier on 2-hydroxyethyl methacrylate ( General information section ) at the European Chemicals Agency (ECHA), accessed on November 17, 2019.
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): 2-hydroxyethyl methacrylate , accessed on March 26, 2019.
