List of ligand abbreviations
Common abbreviations for ligands in chemistry are listed on this page . A ligand is generally understood here as a group of atoms or individual atoms that are coordinated to a central particle. The abbreviations are generally included in the structural formula .
Also amino acids can coordinate as ligands. These can be identified with the three-letter code .
| abbreviation | Surname | maximum teeth (κ) | maximum hapticity (η) | charge | structure |
|---|---|---|---|---|---|
| Acetylacetonate | 2 | −1 |
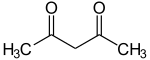
|
||
| Acetonitrile | 1 | 0 |

|
||
| acetate | 1 | −1 |

|
||
| 2- (2-aminoethylamino) ethanol | 3 | 0 |

|
||
| water | 1 | 0 |

|
||
| 2,2'-bis (diphenylphosphino) -6,6'-dimethoxy-1,1'-biphenyl |
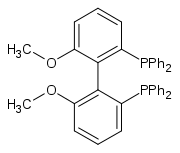
|
||||
| 2,2'-binaphthyldiphenyldiphosphine | 2 | 0 |

|
||
| 1,2-bis [4,5-dihydro-3 H -binaphtho [1,2- c : 2 ′, 1′- e ] phosphhepino] benzene | |||||
| 1,1′-bis {4,5-dihydro-3 H -dinaphtho [1,2- c : 2 ′, 1′- e ] phosphhepino} ferrocene | |||||
| 4,4′-Di- tert- butyl-4,4 ′, 5,5′-tetrahydro-3,3′-bis-3 H -dinaphtho [2,1- c : 1 ′, 2′- e ] phosphhepine | |||||
| 4,5-dihydro-3 H -dinaphtho [2,1- c ; 1 ′, 2′- e ] phosphhepine | |||||
| 1,1'-bi-2-naphthol | 2 | −2 |
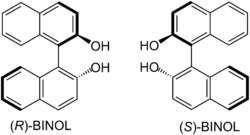
|
||
| 5,5'-bis- tert- butyl-bipyridine | 2 | 0 |

|
||
| 5,5'-bis- tert- butyl-bipyridine | 2 | 0 | |||
| Benzylmethylphenylphosphine | 1 | 0 | |||
| Bis (2 - (( S ) -4- iso- propyl-4,5-dihydrooxazol-2-yl) phenyl) amine | |||||
| Bis (2 - (( S ) -4- tert -butyl-4,5-dihydrooxazol-2-yl) phenyl) amine | |||||
| Bis (oxazoline) ligand (s) | 2 | 0 |

|
||
| 1,2-bis (2,5-diethylphospholano) ethane | 2 | 0 | |||
| Butoxycarbonyl-4-diphenylphosphino-2-diphenylphosphinomethyl-pyrrolidine | |||||
| 2,2'-bipyridine | 2 | 0 |

|
||
| 2,2'-bipyridine | 2 | 0 |

|
||
| Cyclohexyl- o- anisylmethylphosphine | 1 | 0 | |||
| Bis (diphenylphosphino) butane | 2 | 0 |

|
||
| Tropylium (cycloheptatrienyl) | 1 | 7th | +1 |

|
|
| Citrate | 3 | −3 |
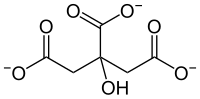
|
||
| 1,5-cyclooctadiene | 2 | 4th | 0 |

|
|
| Cyclooctene | 1 | 2 | 0 |

|
|
| Cyclooctatetraene | 2 | 4th | 0 |
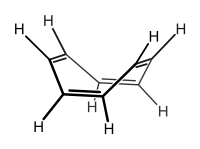
|
|
| Cyclooctatetraene | 1 | 8th | −2 |

|
|
| Cyclopentadienyl | 1 | 5 | −1 |

|
|
| Pentamethylcyclopentadienyl | 1 | 5 | −1 |
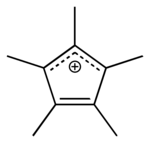
|
|
| Diacetone alcohol | 1 | 0 |

|
||
| 1,4-diazabicyclo [2.2.2] octane | 1 | 0 |

|
||
| Dibenzylidene acetone | 2 | 4th | 0 |
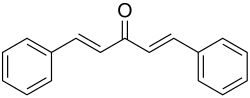
|
|
| Dibenzoyl methane | 2 | −1 |

|
||
| Diethylazodicarboxylate |
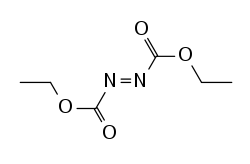
|
||||
| Diethylenetriamine | 3 | 0 |
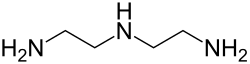
|
||
| O -isopropylidene-2,3-dihydroxy-1,4-bis (diphenylphosphino) butane | 2 | 0 |
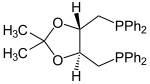
|
||
| (1 R , 2 R ) -Bis [(2-methoxyphenyl) phenylphosphino] ethane | 2 | 0 |

|
||
| 1,2-bis (diphenylphosphino) ethane | 2 | 0 |

|
||
| 4-dimethylaminopyridine | 1 | 0 |

|
||
| Dimethylformamide | 1 | 0 |

|
||
| Dimethylglyoxime | 2 | −1 |

|
||
| 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid | 8th | −4 |

|
||
| 1,2-bis [dimethylphosphino] ethane | 2 | 0 |

|
||
| Neocuproin (2,9-dimethyl-1,10-phenanthroline) | 2 | 0 |

|
||
| Dimethyl sulfoxide | 1 | 0 |

|
||
| (R, R) - & (S, S) -1,2-Diphenylethylene-1,2-diamine | |||||
| 1,2-bis (diphenylphosphino) ethane | 2 | 0 |

|
||
| Bis (diphenylphosphino) methane | 2 | 0 |
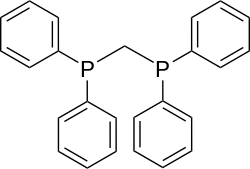
|
||
| 1,3-bis (diphenylphosphino) propane | 2 | 0 |
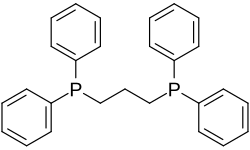
|
||
| Diethylenetriamine pentaacetate | 8th | −5 |

|
||
| Bis (2,5-dimethylphospholano) benzene | 2 | 0 | |||
| Ethylenediaminetetraacetate | 6th | −4 |

|
||
| Ethylene bis (oxyethylene nitrilo) tetraacetate | |||||
| Ethylenediamine | 2 | 0 |

|
||
| α, α, α ′, α′-tetramethyl-1,3-benzenedipropionate (named after Christine G. Espino) | |||||
| Hexafluoroacetylacetone | 2 | −1 |

|
||
| Iminodiacetic acid | 3 | −2 |
|
||
| Ligand | |||||
| 2,2'-bis [( N , N -dimethylamino) (phenyl) methyl] -1,1'-bis (dicyclohexylphosphino) ferrocene | |||||
| N -methyliminodiacetic acid | |||||
| (3,5-Dioxa-4-phosphacyclohepta [2,1-a; 3,4-a '] dinapthalen-4-yl) dimethylamine | |||||
| Methylphenyl- n- propylphosphine | |||||
| 1,3-diketiminate | 2 | −1 |
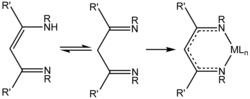
|
||
| Bicyclo [2.2.1] hepta-2,5-dienyl | 2 | 4th | 0 |
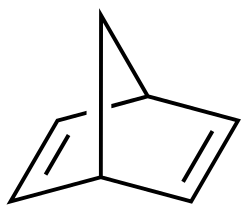
|
|
| Bicyclo [2.2.1] hept-1-yl | |||||
| 2,3-bis (diphenylphosphino) bicyclo [2.2.1] hept-5-ene | |||||
| N -heterocyclic carbenes |

|
||||
| Nitrilotriacetic acid | 4th | −3 |
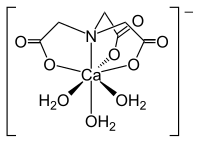
|
||
| acetate | 1 | −1 |

|
||
| tert -butoxide | 1 | −1 |
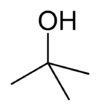
|
||
| Ethanolate | 1 | −1 |
|
||
| Methanolate | 1 | −1 |

|
||
| Oxalate | 2 | −2 |

|
||
| 8-hydroxyquinoline | 2 | 0 |
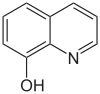
|
||
| Phenyl o -anisylmethylphosphine | |||||
| Tricyclohexylphosphine | 1 | 0 |

|
||
| Phthalocyanine | 4th | −2 |

|
||
| Phenanthroline | 2 | 0 |

|
||
| Picolylamine | |||||
| Piperidine | 1 | 0 |

|
||
| Porphine |
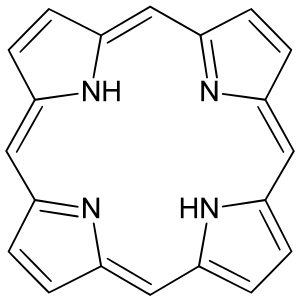
|
||||
| Triphenylphosphine | 1 | 0 |

|
||
| 2-phenylpyridine | 1 | 0 | |||
| Pyridyl | 1 | 0 |

|
||
| Bis (oxazoline) ligand (s) | 3 | 0 |

|
||
| Pyrazine | 1 | 0 |

|
||
| Quinoline-8-olato | |||||
| Bis (salicylidene) ethylene diamine | 4th | −2 |

|
||
| Solvent (solvent) | |||||
| 1,4,7-triazacyclononane | |||||
| α, α, α´, α´-Tetraaryl-1,3-dioxolane-4,5-dimethanol | 2 | −2 |
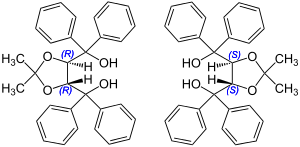
|
||
| Tartrate | 4th | −2 |
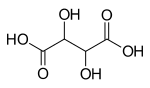
|
||
| Ethylenediaminotriacetate | 5 | −3 | |||
| Terpyridine | 3 | 0 |
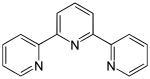
|
||
| Triethylenetetramine | 4th | 0 |
|
||
| Triflate | 1 | −1 |

|
||
| Tetramethylethylenediamine | 2 | 0 |
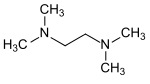
|
||
| Tetramethylethylenediamine | 2 | 0 |

|
||
| Tris (pyrazolyl) borate | 3 | −1 |

|
||
| Tetraphenylporphyrin | 4th | −2 |

|
||
| Triphenylphosphine trisulfonate | |||||
| Tris (2-aminoethyl) amine | 4th | 0 | |||
| Triethylenetetramine | 4th | 0 |
|
||
| Triethylenetetraminehexaacetic acid | 10 | −6 |

|
||
| , | Halide or pseudohalide |
See also
List of abbreviations in organic chemistry
literature
- Nomenclature of Inorganic Chemistry , IUPAC Recommendations 2005 ("Red Book"), Table VII, Ligand abbreviations , p. 267. (PDF file; 4.14 MB)
Individual evidence
- ↑ J. Du Bois et al. J. Am. Chem. Soc. 2004 , 126 , 47, doi: 10.1021 / ja0446294 .












































































































