Methohexital
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Methohexital | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| Brief description |
whitish, odorless, hygroscopic powder, soluble in water (methohexital sodium salt) |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | ||||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
60–64 ° C (methohexital sodium salt) |
|||||||||||||||||||||
| solubility |
Fat soluble, water soluble as methohexital sodium salt |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Methohexital , also methohexiton , is a very short-acting hypnotic (sleep aid) from the range of barbiturates without analgesic (pain-relieving) effects. It is used as an anesthetic .
Indications and use

Methohexital is used primarily for brief anesthesia or for extended anesthesia, followed by the administration of other drugs (such as propofol ). Intravenous injection can be painful, but rectal use can also be used in children . It is used medicinally in the form of the water-soluble sodium salt. At a dose of 1-3 mg / kg body weight, the effect sets in within up to 30 seconds after intravenous injection and lasts for five to ten minutes. In terms of its properties, methohexital is comparable to those of thiopental , which is also a short-acting barbiturate, but it can cause greater excitatory restlessness and the shorter wake-up phase after methohexital is seen as an advantage.
The substance is only used in hospitals or similar institutions under professionally qualified personnel and equipment supervision. It is also a deep sedation or full anesthesia for a surgical procedure or used during dental procedures. Another indication is the long-term sedation of patients with a traumatic brain injury or cerebral edema because of its cerebral pressure lowering effect . However, an excessive reduction in arterial blood pressure by barbiturates must be avoided or compensated for with other medication, as it would lead to a drop in cerebral perfusion pressure (CPP) and thus to a further deterioration in cerebral blood flow. Since methohexital is the only barbiturate that does not increase the seizure threshold , but rather lowers it, it is well suited for anesthesia in electroconvulsive therapy (ECT), which is supposed to trigger a generalized seizure . The patients regain consciousness within three to seven minutes and are back to their original state after about half an hour, which is another advantage of this short procedure.
The drug is also used as a sedative for performing computed tomography and magnetic resonance imaging scans to obtain appropriate images for emergency rooms.
Contraindications
Methohexital is contraindicated in case of hypersensitivity to barbiturates and previous illnesses such as epilepsy . The drug should not be used in asthma or metabolic diseases . Nor is it with simultaneous therapy with warfarin or anti-coagulants .
Pharmacodynamics and pharmacokinetics
Pharmacodynamics : Methohexital extends the opening times of the GABA A receptors . The barbiturate methohexital is able to activate the GABA A receptor, which means that it can be used as a sedative in anesthesia to induce anesthesia . Open ion channels and channel-forming ionophores having pores for chloride ions are permeable. This creates pores or transmembrane channels through which the corresponding chloride ions can diffuse ( ion channels ).
By opening these ion channels, chloride ions flow into the nerve cell. This leads to an (initially local) hyperpolarization of the postsynaptic membrane ( inhibitory postsynaptic potential ).
Pharmacokinetics : The rapid drop in serum levels is due to the rapid breakdown of the lipophilic methohexital in the liver and (less) to redistribution processes within the body. The resulting substances are eliminated via the kidneys . Like other barbiturates (average: 85%), it is predominantly (67–91%) bound to plasma proteins, mainly albumin . The elimination half-life is four hours, but is also given as 3.22 hours and a range of ± two hours. However, it is context-sensitive , i.e. it depends on the dose in which the drug is given and whether this happens once, repetitively or continuously.
The main metabolic pathway of methohexital involves the oxidation of the pentynyl side chain to form 4-hydroxymethohexital. It is not known whether methohexital is excreted in breast milk. The placental barrier is likely to be crossed.
Side effects
As with other barbiturates, intravenous administration of methohexital can cause side effects . Methohexital can cause hiccups and coughs . Involuntary contractions of the muscles or a glottic cramp are also possible . It can also affect lung ventilation. Other side effects can include respiratory depression, respiratory arrest , shortness of breath and cardiac arrest , which can also occur as a result of convulsions , or arterial hypotension and tachycardia .
Possible side effects are high blood pressure , post-narcotic tremors, increased salivation , hyperactivity of the skeletal muscles, seizures, restlessness, anxiety (due to possible postoperative pain), headache, nausea, vomiting, abdominal pain, confusion , redness , itching, hives , hay fever and other acute allergic reactions . When administering a can acute thrombosis or pain at the injection site and the damage to neighboring nerves occur.
synthesis
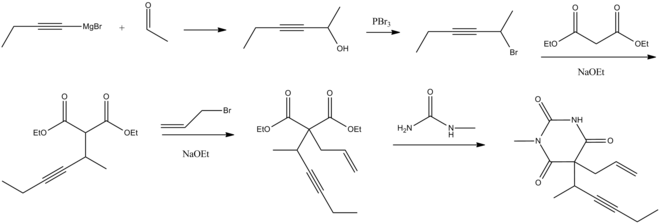
Methohexital can be produced from N -methyl urea and a doubly C-substituted malonic acid ester .
By alkylation of diethyl malonate , starting with 2-bromo-3-hexyne, the (1-methyl-2-pentynyl) -malonsäureester formed. It is then alkylated again with allyl bromide . The final reaction with N -methyl urea leads to methohexital.
WJ Doran patented the synthesis process in the USA in 1959. An enantioselective synthesis has existed since 2001 .
Trade names
Brevimytal (Germany), Brietal (Austria), Brevital (United States, Canada)
End of availability
The pharmaceutical company Hikma Pharmaceuticals announced at the beginning of 2019 that the manufacturer of the active ingredient had discontinued the production of methohexital sodium, the active ingredient of Brevimytal and Brietal, and that it was unable to find another suitable manufacturer. Therefore, the production of the drug was stopped and it was recommended to switch to thiopental if necessary .
literature
- Charlotte Lehmann: The ultra-short narcotic methohexital. Springer-Verlag, 2013, ISBN 978-3-642-46276-4 .
Web links
- Specialist information: Brevimytal Hikma (PDF) .
Individual evidence
- ↑ a b c d Entry on methohexital sodium. In: Römpp Online . Georg Thieme Verlag, accessed on April 23, 2016.
- ↑ There is not yet a harmonized classification for this substance . A labeling of methohexital in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), which was accessed on April 26, 2016, is reproduced from a self-classification by the distributor .
- ↑ Entry on methohexital in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ E. Jessop, RM. Grounds; M. Morgan; J. Lumley: Comparison of infusions of propofol and methohexitone to provide light general anesthesia during surgery with regional blockade. In: Br J Anaesth , Vol. 57, No. 12, December 1985, pp. 1173-1177, PMID 3878718 .
- ↑ N. Mackenzie, IS. Grant: Comparison of propofol with methohexitone in the provision of anesthesia for surgery under regional blockade. In: Br J Anaesth, Vol. 57, No. 12, December 1985, pp. 1167-1172, PMID 3878717 .
- ^ A b c Hans Walter Striebel: Anesthesia - Intensive Care Medicine - Emergency Medicine . Schattauer Verlag, Stuttgart 2012, ISBN 978-3-7945-2890-5 , p. 51 ( limited preview in Google Book search).
- ↑ Lothar Ullrich (ed.): Thiemes intensive care and anesthesia . Georg Thieme Verlag, 2005, ISBN 978-3-13-130910-5 , p. 475 ( limited preview in Google Book search).
- ↑ AO. Hubbell: Methohexital sodium anesthesia for oral surgery. In: J Oral Surg Anesth Hosp Dent Serv, Volume 18, July 1960, pp. 295-298, PMID 14403670 .
- ^ V. De Ocampo: Methohexital sodium anesthesia in oral surgery. In: Dent Mirror (Quezon City), Vol. 3, No. 2, 1966, pp. 9-11, PMID 5228034 .
- ^ GC. Lantz: Regional anesthesia for dentistry and oral surgery. In: J Vet Dent , Vol. 20, No. 3, Sep 2003, pp. 181-186, PMID 14705435 .
- ↑ D. McDonald: Methohexitone in dentistry. In: Aust Dent J , Vol. 25, No. 6, December 1980, pp. 335-342, PMID 6940532 .
- ↑ D. McDonald: Methohexitone in dentistry. Scientific results of 4,379 administrations. 5: Complications. In: Dent Anaesth Sedat , Vol. 11, No. 2, Aug 1982, pp. 51-57, PMID 6962091 .
- ↑ a b c d Michael Freissmuth, Stefan Böhm: Pharmacology and toxicology: From the molecular basis for pharmacotherapy . Ed .: Stefan Offermanns. Springer Verlag, Heidelberg 2012, ISBN 978-3-642-12354-2 , p. 249–250 ( limited preview in Google Book search).
- ↑ Lothar Ullrich (ed.): Thiemes intensive care and anesthesia . Georg Thieme Verlag, Stuttgart 2005, ISBN 978-3-13-130910-5 , p. 387 ( limited preview in Google Book search).
- Jump up ↑ P. Lihua, M. Su, W. Ke, P. Ziemann-Gimmel: Different regimens of intravenous sedatives or hypnotics for electroconvulsive therapy (ECT) in adult patients with depression. In: The Cochrane database of systematic reviews. Volume 4, 2014, pp. CD009763, doi: 10.1002 / 14651858.CD009763.pub2 , PMID 24723301 .
- ^ H. Schulgasser, A. Borowitz: Methohexital anaesthesia in electroconvulsive therapy . In: The South African Medical Journal (SAMJ). Volume 37, Cape Town (Cape Town) 1963, p. 870 ( online ).
- ↑ ES. Pomeranz, CR. Chudnofsky; TJ. Deegan; MM. Lozon; JC. Mitchiner; JE. Weber: Rectal methohexital sedation for computed tomography imaging of stable pediatric emergency department patients. In: Pediatrics , Vol. 105, No. 5, May 2000, pp. 1110-1114, PMID 10790471 .
- ↑ MA. Manuli, L. Davies: Rectal methohexital for sedation of children during imaging procedures. In: Am J Roentgenol , Volume 160, No. 3, March 1993, pp. 577-580, PMID 8430557 .
- ^ A. Arduini, MG. Arduini: Effect of drugs and metabolic alterations on brain stem arousal mechanism . In: J Pharmacol Exp Ther , Vol. 110, No. 1, January 1954, pp. 76-85, PMID 13118481 .
- ↑ Bertram George Katzung: Basic and Clinical Pharmacology , 10th Edition, Lange Medical Books / McGraw-Hill, New York 2007, pp. 406-407 ( online )
- ↑ a b c Entry on methohexital at Vetpharm, accessed on April 20, 2016.
- ↑ B. Bally, JF. Payen; F. Serre-Debeauvais; B. Tranchand; M. Gavend; P. Stieglitz: Pharmacokinetics of methohexital given by constant rate intravenous infusion In: Ann Fr Anesth Reanim , Volume 11, No. 2, 1992, pp 136-140, PMID 1503284 .
- ↑ BN. Sverdlov, FO. Holley: Intravenous anesthetic agents. Pharmacokinetic-pharmacodynamic relationships ., In: Clin Pharmacokinet , Vol. 12, No. 2, February 1987, pp. 79-110, PMID 3549105 .
- ↑ JW. Sear: General kinetic and dynamic principles and their application to continuous infusion anaesthesia ., In: Anaesthesia , 38 Supplement, July 1983, pp. 10-25, PMID 6346936 .
- ↑ H. Phillips, A. Brown; IH. Koven: Coincidental laryngospasm and duodenal perforation during general anesthesia for exodontia. Report of case. In: J Can Dent Assoc (Tor), Vol. 35, No. 11, Nov. 1969, pp. 603-605, PMID 5259696 .
- ↑ TA. Yemen, J. Pullerits; R. Stillman; M. Hershey: Rectal methohexital causing apnea in two patients with meningomyeloceles . In: Anesthesiology, Vol. 74, No. 6, June 1991, pp. 1139-1141, PMID 2042766 .
- ↑ MA. Rockoff, NG. Goudsouzian: Seizures induced by methohexital . In: Anesthesiology , Vol. 54, No. 4, April 1981, pp. 333-335, PMID 7212335 .
- ↑ K. Metriyakool: Seizures induced by methohexital . In: Anesthesiology , Vol. 55, No. 6, December 1981, p. 718, PMID 7305065 .
- ↑ T. Loddenkemper, G. Möddel; SU. School; E. Wyllie; HH. Morris: Seizures during intracarotid methohexital and amobarbital testing . In: Epilepsy Behav , Volume 10, No. 1, February 2007, pp. 49-54, PMID 17049312 .
- ↑ JM. Millar, AM. Barr: The prevention of pain on injection. A study of the effect of intravenous lignocaine before methohexitone . In: Anaesthesia , Vol. 36, No. 9, September 1981, pp. 878-880, PMID 7030121 .
- ↑ KH. Simpson, PJ. Halsall; Approx. Sides; JF. Keeler: Pain on injection of methohexitone. The use of lignocaine to modify pain on injection of methohexitone during anesthesia for electroconvulsive therapy. In: Anaesthesia . Vol. 44, No. 8, Aug 1989, pp. 688-699, PMID 2782576 .
- ↑ RS Vardanyan and VJ Hruby: Synthesis of Essential Drugs , Elsevier 2006, p. 10 ( online ( memento of the original dated February 26, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and Archive link according to instructions and then remove this note. ).
- ↑ H. Brunner: Narcotic drug methohexital: synthesis by enantioselective catalysis. In: Chirality . Volume 13, Number 8, August 2001, pp. 420-424, doi: 10.1002 / chir.1054 , PMID 11466761 .
- ↑ newsletter for non-delivery of Brevimytal Hikma . Communication from the Federal Institute for Drugs and Medical Devices dated January 7, 2019.
- ↑ Information letter on Brevimytal Hikma - January 2019 . Yellow list on January 10, 2019.