Oxymetazoline
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
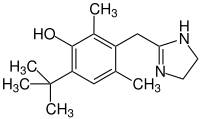
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Oxymetazoline | ||||||||||||
| other names | |||||||||||||
| Molecular formula | C 16 H 24 N 2 O | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| Mechanism of action | |||||||||||||
| properties | |||||||||||||
| Molar mass | 260.37 g · mol -1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
181-183 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Oxymetazoline is a chemical compound from the group of imidazoline derivatives. It is used as a medicinal substance to reduce the swelling of the nasal mucosa . As α-sympathomimetic it is an α 1 -adrenoceptor - agonist and causes the contraction of smooth muscle . As a result, the locally located blood vessels in the nose are narrowed ( vasoconstriction ) and the reduced blood flow causes the mucous membranes to swell. Oxymetazoline is therefore used in nasal sprays , nasal ointments and nasal drops .
In ophthalmology , the drug is used in eye drops to treat non-infectious and allergic forms of conjunctival irritation or conjunctivitis (conjunctivitis).
presentation
Oxymetazoline was first synthesized by Wolfgang Fruhstorfer and Helmut Müller-Calgan at E. Merck Aktiengesellschaft in Darmstadt on the basis of xylometazoline as the starting point. During the work it was found that the majority of the new derivatives in the nose were more nasal than relieving. However, some showed the effect of xylometazoline. From these, the derivative named generically oxymetazoline was selected, which is characterized by a longer duration of action compared to the existing xylometazoline. That is why the drug was selected for the on-board pharmacy for the first moon landing in 1969. It is represented by the reaction of 2,6-dimethyl-3-hydroxy-4- tert- butyl-benzyl cyanide with ethylenediamine in the presence of carbon disulfide and with the release of ammonia at 100 ° C. for 48 hours. Oxymetazoline is then obtained from the reaction product by repeated recrystallization from benzene with a yield of 75%.
Effects
For decades only the decongestant effect of oxymetazoline was known, which is achieved by narrowing small blood vessels in the nasal mucous membrane. In the last few years, a causal effect against rhinitis has also been proven. Oxymetazoline has a direct antiviral effect and reduces the expression of ICAM-1 , a receptor for the human rhinovirus . As a result, the rhinoviruses, the most common pathogen causing acute rhinitis, find fewer entry points into the nasal mucosa. The release of pro-inflammatory cytokines such as interleukins and TNF-α is also prevented in the presence of oxymetazoline. Oxidising leukotrienes are suppressed by oxymetazoline, but anti-inflammatory leukotrienes are not. Oxymetazoline shortens the duration of an acute cold by a third.
Side effects
Prolonged use can lead to the drying out of the nasal mucous membranes and result in increased blood flow in the corresponding vessels ( congestion ). Therefore a therapy duration of seven days should not be exceeded. Occasionally there is palpitations or an increase in blood pressure. Due to the systemic effect, use during pregnancy and breastfeeding is only recommended after medical advice. Oxymetazoline is well tolerated by babies and toddlers in an age-appropriate dosage.
Trade names
Dr. Cabana nasal drops (A), Nasivin (D, A, CH), Vicks Sines (CH), Wick Sinex (D, A)
Individual evidence
- ↑ a b entry on oxymetazoline. In: Römpp Online . Georg Thieme Verlag, accessed on May 26, 2014.
- ↑ a b Data sheet Oxymetazoline hydrochloride from Sigma-Aldrich , accessed on April 16, 2011 ( PDF ).
- ↑ Summary of Product VISTOXYN ® Liquifilm the company PHARM-ALLERGAN GmbH.
- ↑ Process for the production of a new imidazoline derivative. Auslegeschrift no. 1117588 of the German Patent Office on November 31, 1961.
- ^ S. Koelsch, M. Tschaikin, F. Sacher: Anti-Rhinovirus-specific Activity of the Alpha-sympathomimetic Oxymetazoline . In: Arzneimittelforschung (Drug Research) 2007; 57 (7): 475-482.
- ↑ A. Tuettenberg, S. Koelsch, J. Knop, H. Jonuleit: Oxymetazoline modulates proinflammatory cytokines and the T-cell stimulatory capacity of dendritic cells . In: Experimental Dermatology 2007; 16, 171-178.
- ^ I. Beck-Speier, N. Dayal, E. Karg, KL Maier, G. Schumann, M. Semmler and SM Koelsch: Oxymetazoline Inhibits Proinflammatory Reactions: Effect on Arachidonic Acid-Derived Metabolites . In: Journal of Pharmacology and Experimental Therapeutics 2006, 316: 843-851.
- ↑ S. Reinecke, M. Tschaikin: Investigation of the effectiveness of oxymetazoline on the duration of the cold . In: MMW 2005 (147th year): 113–118.
- ↑ Instructions for use for Nasivin ® from Merck Selbstmedikation GmbH.
- ↑ M. Tchaikin. Cold treatment for babies and toddlers. Deutsche Apotheker Zeitung 2005, 44 (145th year): 97-98.