Polyhydroxybutyric acid
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|

|
|||||||
| General | |||||||
| Surname | Polyhydroxybutyric acid | ||||||
| other names |
|
||||||
| CAS number | 26063-00-3 | ||||||
| Monomer | ( R ) - 3-hydroxybutanoic acid | ||||||
| Molecular formula of the repeating unit | C 4 H 6 O 2 | ||||||
| Molar mass of the repeating unit | 86.09 g mol −1 | ||||||
| PubChem | 13061653 | ||||||
| Type of polymer | |||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| density |
1.2-1.25 g / cm³ |
||||||
| Melting point |
approx. 175 ° C |
||||||
| Glass temperature |
0-5 ° C |
||||||
| solubility |
|
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
The biopolymer polyhydroxybutyric acid (other names: polyhydroxybutyrate , PHB , poly (R) -3-hydroxybutyrate , P (3HB) ) is a polyhydroxyalkanoate (PHA). 3-Hydroxybutyric acid is the monomer of polyhydroxybutyric acid. It contains a hydroxy group at one end and a radical methyl group at the other end of the alkyl . There is a stereocenter on the β- carbon atom of the monomer 3-hydroxybutyric acid, the compound is optically active and is usually in the ( R ) configuration. The ( S ) isomer and the racemate [( RS ) configuration] are of little importance.
PHB is a polyester that can be produced by fermentation from renewable raw materials . The polyol ester PHB is isotactic and absolutely linear. It belongs to the group of thermoplastic polyesters and can therefore be deformed when heated.
Biogenic production
It was first isolated and characterized in 1925 by the French microbiologist Maurice Lemoigne (born December 16, 1883 in Paris, † May 9, 1967 ibid). It is accumulated as a storage material in a variety of microorganisms , including Cupriavidus necator .
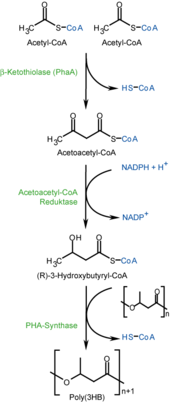
PHB is synthesized as an energy store during the assimilation of carbon, mainly in the form of glucose and starch , and metabolized again in the absence of other energy sources. The biosynthesis of PHB by bacteria generally takes place in three steps, which are catalyzed by three enzymes : Two molecules of acetyl-CoA condense in a Claisen condensation through catalysis of β- ketothiolase to acetoacetyl-CoA, which in a stereospecific reaction by the NAD (P) H-dependent acetoacetyl-CoA reductase is reduced to ( R ) -3-hydroxybutyryl-CoA. This serves the PHB synthase as a substrate for the polymerization to the PHB. The poly-3-hydroxybutyrate (P3HB) form of PHB is probably the most common form of the polyhydroxyalkanoates. A number of other polymers of this class, polyhydroxyalkonates, are produced by various organisms, including poly-4-hydroxybutyrates (P4HB), polyhydroxyvalerates (PHV), polyhydroxyhexanoates (PHH), polyhydroxyoctanoates (PHO) and their copolymers . The polymers are extracted from the cell material of the bacteria as powder or granules.
Already in the 1980s, the three genes responsible for PHB production were transferred from Alcaligenes eutrophus to the more easily manipulated Escherichia coli by genetic engineering . In addition, PHB genes were transferred to garden cress at Michigan State University . The transgenic plants produced up to 14% PHB in the dry matter of the leaves.
A special strain of the Acidovorax bacterium is able to break down PHB with the help of the enzyme D-beta-hydroxybutyrate dehydrogenase .
The molar mass of the polymer is between 50,000 and 1,000,000 g / mol. The highest molar mass is reported with 20,000,000 g / mol.
Extraction
The fermentative synthesis can be based on sugar ( glucose ) and starch , but is also made from other nutrients such as glycerine and vegetable oil such as. B. palm oil possible. PHB is isolated from the bacterial cells by extraction.
Fermentative extraction from sugar and starch
Grace and Imperial Chemical Industries (ICI) began industrial development in 1960/1976. Chemie Linz and PCD Polymer GmbH introduced (1982/1988) a new strain that was able to enrich the polymer during the growth phase. In 1983 MJ de Smet found that the bacterium Pseudomonas oleovorans produces PHB by using octane as a nutrient . In 1988, the Alicaligenes eutrophus gene was cloned and implemented in the fast-growing Escherichia coli bacterium . In 1993, Urs J. Hänggi acquired bacterial strains and know-how from PCD and founded Biotechnology Co. under the brand name Biomer.
Fermentative production from glycerine
The "SECI, Holding of the industrial group Maccaferri" and "Bio-on" aim to produce bioplastics based on PHA from glycerine , which is a by-product of the production of biodiesel . It is specifically about the construction of a plant with an annual capacity of 5,000 tons, expandable to 10,000 tons. Glycerin-based PHB is no different from glucose-based PHB. Zobellella denitrificans can be used as a bacterium .
The bacterium Burkholderia cepacia ATCC 17759 produces PHB from nutrient media with different glycerine contents of 3 - 9% by volume. In a 200 l fermentation, a yield of 23.6 g / l of dry biomass was achieved with a yield of 7.4 g / l of PHB.
The bacterium Pandoraea sp. Isolated in the Brazilian rainforest . MA03 produces PHAs from glycerine from the biodiesel industry.
Further PHB extraction processes
In research on microbial electrosynthesis , microorganisms are used at the cathode that produce the polymer BioElectroPlast polyhydroxybutyric acid (PHB) from flue gas, air and electricity from renewable sources.
PHB copolymers
Pure PHB can only be used to a limited extent as it is more brittle than polypropylene. Only with the biochemical synthesis of copolymers, such as. B. Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) , (3PHB-3HV) it could be used more flexibly. The first biodegradable shampoo bottle made from PHBV (CAS number: 80181-31-3) was manufactured in 1975. Ralstonia eutropha was the bacterium used for biosynthesis.
A PHB polyvinyl chloride (PVC) copolymer was developed by "Metabolix". The polyhydroxyalkanoate (PHA) "Mirel" causes tougher plasticization and improved processing steps. It also has high UV stability and transparency . It is resistant to fungi . Because of its good miscibility with PVC, the PHA modifier does not migrate, evaporate and is not extracted. The PHB additive can replace previous, sometimes unstable additives in PVC. The copolymer can be processed under the same conditions as PVC.
The company "TianAn Biologic Material Co., Ltd.", China (Beilun District, Ningbo City, Zhejiang Province) sells the copolymer polyhydroxy-butyrate-co-valerate (PHBV) (CAS number: 80181) under the trade name “Enmat” -31-3) as powder or granulate, pellets. It is obtained by fermentation with the unmodified Ralstonia Entropha bacterium from the glycose of the corn starch. After fermentation, it is separated from the bacteria by cold water extraction.
The company "PHB Industrial", Brazil (Serrana, Sao Paulo), produces PHB from sucrose by fermentation. The plant produced 50 t / year in a pilot plant in 2010. The aim is to increase the production capacity in a production facility to 100,000 t / year.
Siemens, BASF, Technical University of Munich and University of Hamburg have developed a 70% green polymer that contains PHB. It has the potential to replace polystyrene- based acrylonitrile butadiene styrene (ABS).
PHB blends
PHB is also used for blends , i.e. for mixtures with other polymers. For example, the addition of cellulose acetates can achieve special material properties. The range of properties of the blends ranges from adhesives to hard rubber. Instead of cellulose acetate, starch, cork and inorganic materials are also conceivable as additives. Mixing with cheap additives (cellulose acetate is a cheap product, mainly for cigarette filter production) also has a positive effect on the production costs of the blends. In the medium term, according to numerous researchers, this will reduce manufacturing costs down to the area of petroleum-based plastic materials.
The addition of 5% PHB to polylactic acid (PLA) improves the toughness of injection molded parts and films. In film applications, PLAa / PHA films have a tensile strength comparable to HDPE . This allows lower strengths with a higher load capacity and constant tear resistance . Even small amounts of PHB in PLA improve the suppleness of fibers. They create a soft, silky feel in woven and non-woven applications.
Polyvinyl acetate improves the physical properties of PHB and makes processing easier. Depending on the polyvinyl acetate content and type, for example, the crystallization can be optimized or the melting point lowered. Thanks to the improved property profile and the high heat resistance of PHB, applications in the hot filling area are possible.
Custom-made plastics can also be seen as a blend between cheap organic macromolecules such as B. Imagine starch, wood, straw and PHB. They could be used as packaging material in agriculture.
properties
PHB can be processed thermoplastically as granules . Compared to the petrochemically produced plastic polypropylene (PP), it has similar properties in terms of melting temperature , crystallinity , glass transition temperature and tensile strength . It is harder and more brittle compared to PP. Copolymers, blends or plasticizers are used to increase its flexibility.
General material properties
- Density 1.2-1.25 g / cm³
- Melting point approx. 175 ° C
- Glass transition temperature 0-5 ° C
- Enthalpy of fusion (kJ / kg) 113
- from 200 ° C it begins to decompose
- insoluble in water and relatively stable against hydrolysis (in contrast to most other biopolymers)
- waterproof, even against hot liquids> 120 ° C
- biodegradable . Microbial enzymes can break down the macromolecule into smaller units
- non- toxic and biocompatible and therefore suitable for medical applications.
- Suitable for contact with food in food packaging.
- microwaveable
- processable like classic thermoplastics
- behaves like liquid crystal polymer (LCP) in the melt . It is therefore suitable for injection molding with fine structures, thin walls and for micro parts
- Film properties and rheological properties comparable to LDPE
- Highly crystalline (60 to 70%), therefore good solvent resistance
- Low tendency to creep .
- permeable to oxygen similar to PP
- For a PHB copolymer with 8 mol% PHV (PHB 92 / PHV 8), the permeability for oxygen at 25 ° C is: 0.1–0.2 (× 10 −13 (volume cm 3 ). (Per area cm −2 ). (per time s −1 ). (per pressure Pa −1 ) and (times film thickness cm)
- The permeability for water at 38 ° C: 1000-2000 (x10 −13 (volume cm 3 ). (Per area cm −2 ). (Per time s −1 ). (Per pressure Pa −1 ) and (times film thickness cm ) ie the higher the pressure and the lower the film thickness, the more oxygen or water permeates per area through the film.
- Moisture absorption 0.4-0.75%.
- Chemically resistant similar to PET : satisfactory with alcohols; good for fats and oils; bad with alkalis and acids ; satisfactory with dilute acids.
- Satisfactory resistance to ultraviolet radiation (UV).
- Inherently stable
- Expansion at break 5–15%
- Colorable.
- piezoelectric .
- printable and stickable.
- Soluble in chloroform and dichloromethane , moderately in 1,2-dichloroethane and aniline .
Mechanical properties of substances
(The values are within a certain range of fluctuation depending on the PHB content).
| property | PHB |
|---|---|
| Module (MPa) (1 mm / min) | 840-1500 |
| Tensile Strength (MPa) (50 mm / min) | 15–30 (similar to polypropylene ) |
| Elongation at break (%) (50 mm / min) | 8-15 |
| Bending stress (N / mm 2 ) | 18-35 |
| Bending stress 3.5% (N / mm 2 ) | 16-29 |
| Outer fiber strain (%) | 2.5-6.6 |
| Impact strength 23 ° C (KJ / m2) (ISO 179 / 1eU) | no break |
| Impact strength −30 ° C (KJ / m2) (ISO 179 / 1eU) | 30-70 |
| Notched impact strength 23 ° C (ISO 179 / 1eA) | 2.7-4.7 |
| Notched impact strength -30 ° C (ISO 179 / 1eA) | 2.7-4.7 |
| Hardness (Shore D) | 57-69 |
| Shrinkage (%) | 1.2-1.3 |
Properties of PHB / PHV copolymers
| property | PHV | (PHB 97 / PHV 3) | (PHB 92 / PHV 8) | (PHB88 / PHV 12) | (PHB75 / PHV 25) |
|---|---|---|---|---|---|
| Copolymer (mol%) | 100 | 8th | 12 | 25th | |
| shape | Movie | Granules | |||
| Thermal properties | |||||
| Melting point (° C) | 110 | 170 | 165 | 148 | 127 |
| Glass transition temperature (° C) | 8th | 6th | 4th | minus 6 | |
| Enthalpy of fusion (kJ kg −1 ) | 84 | 99 | 92 | 80 | 54 |
| Specific heat (JK −1 kg −1 ) | 1400 | 1400 | |||
| Thermal conductivity at 23 ° C (W m −1 K −1 ) | 0.15 | 0.15 | |||
| Electrical Properties | |||||
| Dielectric constant at 1 MHz | 3 | 3 | |||
| specific volume resistance (ohm cm) | 10 −16 | 10 −16 | |||
| Mechanical properties | |||||
| E-modulus in tensile test (GPa) | 2.9 | 1.0-19 | 1.5 | 0.7 | |
| Notched impact strength according to Izod (J m −1 ) | 60 | 95 | 120 | 400 | |
| Elongation at break (%) | 35 | 35 | |||
| Tensile strength (MPa) | 38 | 25-30 | 35 | 30th |
Biodegradability
The temporal development of research from 1964 to 1997 on PHA and in particular on PHB degradation is shown in Milan Matavulj et al. (2000) listed.
PHBV does not decompose in humid air, which guarantees a long service life as a packaging material.
PHB can be broken down in nature by bacteria, fungi or algae. The rate of degradation depends on the environmental conditions and the thickness of the material. PHB can also be broken down by hydrolysis, mechanical, thermal, oxidative or photochemical stress. It is the hydrolytic fraction that enables its use in medicine.
With warm composting as in composting plants, a 288 µm film (Mvera B5002) is completely degraded within 10 weeks. A film only 25.5 µm thick will break down in about 2 weeks.
The degradation of a 50 µm film of PHBV takes 10 weeks in cold soil. Under anaerobic conditions, a 50 µm film of PHBV can be completely degraded within 1 to 2 weeks in brackish water or within 7 weeks under aerobic conditions. The degradation takes 15 weeks in lake water.
Applications
General uses
Polyhydroxybutyrate (PHB) is used in pure form or as copolymers or in blends : for contact with food (foils, wrappings, bowls, cutlery); stretchable or shrinkable packaging or as compostable carrier bags and as a mulch film; Films for laminating and coating paper cups, plates or non-woven fibers.
Its melt can be injection molded such. B. for cosmetic bottles , cups , irrigation systems or as a fluid for 3D printers for complex structures.
In pharmacy and medicine, it is used for packaging medicines, for medical bone augmentation and as implants and artificial esophagus .
In electronics, it tries to replace conventional plastic such as polyethylene (PE-LD).
Further areas of application
"Run bubbles" comparable to PE-LLD .
processing
Processing in injection molding
The company "Biomer" in Krailling (Germany) sells PHB thermoplastics for high-quality injection molded parts made from renewable raw materials. The PHB granulates can be processed on standard injection molding machines. The melt behaves like liquid crystalline material (LCP). The absence of any branches in the polymer chains (absolutely linear) and the absence of long side chains lead to a melt that is thin. This is why thin-walled parts or parts with a complex structure and parts with a fine surface (<1μ) can also be injected on small machines with a PHB melt. The tacticity of the molecules (absolutely isotactic, absolutely stereoregular) allows fast cycle times. This is particularly interesting for the injection of micro parts, where machine running times are the main cost factor. The absence of any nucleating agents makes it possible to adjust the brittleness of injection molded parts almost at will.
Further processing methods
Further processing methods are thermoforming , sheet and film extrusion , blown film processes on standard machines .
Industrial manufacturing
List of production companies:
| company | Trade name | product | Country, (place, region) | Raw material | Capacity (tons / year) |
|---|---|---|---|---|---|
| BASF | PHB, PHBV blend with Ecoflex | Germany | Strength | ||
| Biocycle PHA Industrial | P3HB | Brazil (Serrano) | Sugar cane | 100 | |
| Biomatera | Biomatera | PHBV, PHA resins | Canada | Sugar, renewable raw materials | |
| Biotechnology Co., Biomer | Biomer P209, P226, P300, P304, P316 | PHB granules, PHB balls | Germany, (Krailling) | Sucrose | 50 |
| Bio-On | Minerv Bio Cosmetics | PHA | Italy, (Castel San Pietro Terme near Bologna) | Various agricultural products, agricultural waste | 19 (2019) |
| Blue PHA | P4HB, P3HP, PHV, P3HP3HB, P3HP4HB | China | |||
| BTF, Austria | PHB | Austria | 20-100 | ||
| Chemistry Linz | PHB | Austria | 20-100 | ||
| Danimer Scientific | Novax PHA | mcl-PHA | United States | Cold pressed canola oil | |
| Goodfellow Cambridge Ltd | Goodfellow | PHA, PHB-PHV (88% - 12%) | England (Cambridge) | ||
| Imperial Chemical Industries (ICI) | Biopoly | PHBV | United Kingdom (Billingham) | 300,000 | |
| Jiangsu Nantian Group | Jiangsu Nantian | P3HB | China | ||
| Kaneka Corporation and P&G Chemicals | AONILEX, Kaneka | 3-PHB, PHBH, PHBHHx | Japan (Osaka) | Vegetable oils | 3500 expansion to 50,000 / 2020 |
| Meredian | Biopolymers | United States | 272, expansion to 272,000 / 2020 | ||
| MHG formerly P&G Chemicals | Nodax, Nodak | PHBH, PHA | USA, (Bainbridge, Georgia) | Cereals, sugar beet, vegetable oil, canola oil | 10,000 |
| Metabolix Inc. and BP | Mirel | PHA | USA (Woburn, MA) | Grain | 50,000 |
| Mitsubishi Gas Chemical Company | Biogreen | P (3HB) | Japan | Methanol from (CO 2 ) and (H 2 ) from exhaust gas | 10,000 |
| Monsanto | PHB, PHBV | United States | |||
| Newlight Technologies LLC | PHA resins | United States | Air carbon, methane emissions | ||
| PHB Industrial | Biocycle | PHB, PHBV, | Brazil, (Serrana, Sao Paulo) | Sugar cane, ethanol, sucrose | 30,000–50,000 to be expanded to 100,000 t / year |
| PolyFerm | VersaMer | mcl-PHA, PHA | Canada | Sugar, vegetable oils | 3,000 |
| Polyscience. Inc. | PHB | United States |
|
||
| P&G Chemicals | Nodax | P3HB | USA / Japan | ||
| Siemens, BASF, Technical University of Munich, University of Hamburg | Green polymer with 70% PHB | Germany (Munich, Hamburg) | Palm oil, starch | ||
| Shenzen Ecomann Biotechnology Co. Ltg | Ecomann Biosesin | PHA granules | Sugar, glucose | 5,000 | |
| Sirim Bioplastic | PHA various | Malaysia (Shah Alam, Selangor) | Palm oil, palm oil methyl ester, palm kernel oil | 2,000 | |
| Tepha Medical Devices | TephaFlex | PH4B | United States | ||
| Telles LLC joint venture between Archer Daniels Midland Company and Metabolix | Metabolix, Mvera | PHB-PLA copolymers, multiple PHAs with three to 6 carbons | USA, (Clinton Iowa) | Glucose / sugar from grain | 50,000 / 2010, expansion to 500,000 t / 2020 |
| Tepha | Tephaflex, TephElast | United States | |||
| Tianjin GreenBio Material Co. | GreenBio, Sogreen | P (3HB-4HB) films, granules, foam granules | China (Binhai District in Tianjin) / Netherlands | sugar | 10,000 |
| Tianjin Northern Food | PHA | China | 10 | ||
| Tianzhu | Tianzhu | PHBH | China | ||
| TianAn Biologic Materials Co .; Zhejiang; TianAn biopolymer | Ecomat, Enmat | PHB, PHBV | China, (Beilun District, Ningbo City, Zhejiang Province) | Dextrose / glucose from cereals, tapioca | 10,000 t / 2010, expansion to 50,000 t / 2020 |
| Yikeman ShanDong | P3HB4HB | China | 3000 | ||
| Zeneca PLC formerly Imperial Chemical Industries | Biopoly | PHB - PHBV copolymer | England |
literature
- Hans-Josef Endres, Andrea Siebert-Raths: Technical Biopolymers Hanser-Verlag, Munich 2009, ISBN 978-3-446-42104-2 .
- Astrid Rebischke, Sigrid Jauris-Heipke: Production of bioplastics by Lactobacillus acidophilus .
- A. Steinbüchel: Perspectives for biotechnological production and utilization of biopolymers: Metabolic engineering of polyhydroxyalkanoate biosynthesis pathways as a successful example . Macromol. Bioscience 1: 1-24 (2001) doi : 10.1002 / 1616-5195 (200101) 1: 1 <1 :: AID-MABI1> 3.0.CO; 2-B (free full text).
- Ray Smith Biodegradable polymers for industrial applications , CRC Press, May 17, 2005, limited preview in the Google book search, Chapter 2.3.1. Microbial PHB and PHBV production, pp. 42-44.
Web links
- Polyhydroxyalkanoates (PHA)
- Material data sheet for Biomer P226 - PHB (manufacturer's website )
- Mechanical properties of the PHP Biomer series: P209 (89.2% GnwR), P226 (89.8% GnwR), P300 (92.6% GnwR), P304 (97.9 GnwR), P316 (97.5 GnwR) ( GnwR = content of renewable raw materials) (manufacturer side)
- Biomer, The fermentation, the extraction
- Metabolix
- Biodegradable Polymers, Biodegradable Materials (BAW)
- Upgrading Glycerol, The Production of Polyhydroxybutyric Acid (PHB) in Bacillus megaterium
Individual evidence
- ↑ a b c Mechanical properties of the PHP Biomer series: P209 (89.2% GnwR), P226 (89.8% GnwR), P300 (92.6% GnwR), P304 (97.9 GnwR), P316 (97, 5 GnwR) (GnwR = content of renewable raw materials) (manufacturer side).
- ↑ a b Christian Vogel: Characterization of the thermal and mechanical properties of polyhydroxyalkanoate (PHA) homopolymers, copolymers and polymer blends; Dissertation 2008, page 14, 44 .
- ↑ a b Urs J. Hänggi processing of PHB
- ↑ a b c d e N. Jacquel, et al .: Solubility of polyhydroxyalkanoates by experiment and thermodynamic correlations . In: AIChE J. . 53, No. 10, 2007, pp. 2704-2714. doi : 10.1002 / aic.11274 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ B Senthil Kumar, G Prabakaran: Production of PHB (bioplastics) using bio-effluent as substrate by Alcaligen (e) s eutrophus . In: Indian Journal of Biotechnology . 5, 2006, pp. 76-79.
- ↑ N. Jacquel, et al .: Isolation and purification of bacterial poly (3-hydroxyalkanoates) . In: Biochem. Closely. J. . 39, No. 1, 2008, pp. 15-27. doi : 10.1016 / j.bej.2007.11.029 .
- ↑ Takanashi M, Shibahara T, Shiraki M, Saito T: Biochemical and genetic characterization of a D (-) - 3-hydroxybutyrate dehydrogenase from Acidovorax sp. strain SA1 . In: J. Biosci. Bioeng. . 97, No. 1, 2004, pp. 78-81. doi : 10.1016 / S1389-1723 (04) 70170-X . PMID 16233594 .
- ↑ a b c d e f g h i Ashok Pandey, Rajeshwar D. Tyagi, Jonathan WC Wong: Chapter 1 Bioplastics From solid Waste Table 1.2 . In: Current Developments in Biotechnology and Bioengineering Solid Waste Management . Verlag Elsevier, September 15, 2016, ISBN 9780444636645 .
- ^ Short story by PHB
- ↑ Agreement between Maccaferri and Bio-on for the production of bioplastics from glycerine December 23, 2015
- ↑ Mohammad HA Ibrahim, Alexander Steinbüchel: Poly (3-Hydroxybutyrate) Production from Glycerol by Zobellella denitrificans MW1 via High-Cell-Density Fed-Batch Fermentation and Simplified Solvent Extraction▿ . In: Applied and Environmental Microbiology . 75, No. 19, 2009, pp. 6222-6231. doi : 10.1128 / AEM.01162-09 .
- ↑ Vijay Kumar Garlapati, Uttara Shankar, Amrita Budhiraja: bioconversion technologies of crude glycerol to value added industrial products . In: Biotechnology Reports . 9, No. March 2016, 2016, pp. 9-14. doi : 10.1016 / j.btre.2015.11.002 .
- ↑ Fabricio Coutinho de Paula, Sérgio Kakazu, Carolina Bilia de Paula, José Gregório Cabrera Gomez, Jonas Contiero: Polyhydroxyalkanoate production from crude glycerol by newly isolated Pandoraea sp. . In: Journal of King Saud University - Science . 2016. doi : 10.1016 / j.jksus.2016.07.002 .
- ↑ Karlsruhe Institute of Technology (KIT): Microbes produce bioplastics from flue gas and electricity. Bio-based News, November 8, 2016, accessed November 13, 2016 .
- ↑ Michael Pankratius: Polyhydroxyalkanoate - PHA - Bioplastics - Polyhydroxybutyric acid - PHB, plastic injection molding. Renewable raw materials - The future of the field, August 6, 2010, accessed on November 15, 2016 .
- ↑ Yelena Kann: Modifying PVC with Bio-Based PHA Rubber . In: Plastics Technology . No. July 2013, 2013.
- ↑ TianAn biopolymer Enmat
- ↑ Fernanda de Biagio: PHB Industrial seeking partner for biopolymer production. November 30, 2010, accessed November 15, 2016 .
- ^ Modification of PLA with Mirel a-PHA copolymer .
- ↑ PHA / PHB applications from Wacker
- ^ A b Robert H. Marchessault1, Ga-er Yu2 Crystallization and Material Properties of Polyhydroxyalkanoates
- ↑ a b c Polyhydroxybutyrate / Polyhydroxyvalerate 8% biopolymer film (PHB92 / PHV 8)
- ↑ a b c d Stan Haftka: Mirel PHB - Bio based Plastics with Performance and Biodegradability. Mirel Bioplastics by Telles, October 24, 2011, accessed November 24, 2016 .
- ↑ Material properties and mechanical data of the PHB polymer (manufacturer's website)
- ↑ OFI bioplastics overview and current trends in the bioplastics world
- ↑ Milan Matavulj; Novi Sad; Hans Peter Molitoris: Biodegradation of polyhydroxyalkanoate-based plastic (BIOPOL) under different environmental , Hoppea, Denkschr. Rain. Bot. Ges. 61, Bresinsky-Festschrift (2000): 735-749
- ↑ Urs J. Hanggi Properties and processing of PHB formulations
- ↑ Ashok Pandey, Sangeeta Negi, Carlos Ricardo Soccoi: Chapter 2 Genesis of Renewable Plastics and Integration in the Plastic Stream Table 2.3 . In: Current Developments in Biotechnology and Bioengineering, Production, Isolation and Purification of Industrial Products . VERLAG Elsevier, September 17, 2016, ISBN 978-0-444-636737 , p. 49.
- ↑ Michel Biron: Chapter 2.6, List of Commercial Offer Examples, Table 2.3 . In: Industrial Applications of renewable Plastics, Environmental, Technological, and Economic Advances . Elsevier, November 10, 2016, p. 48.
- ↑ Companies Producing PHA for Bioplastics and Using PHA in applications
- ↑ Bio-on opens PHA plant in Italy near Bolognia
- ↑ C. Kourmentza, J. Plácido, N. Venetsaneas, A. Burniol-Figols, C. Varrone, HN Gavala, MA Reis: Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. In: Bioengineering. Volume 4, number 2, June 2017, p., Doi : 10.3390 / bioengineering4020055 , PMID 28952534 , PMC 5590474 (free full text) (review).
- ↑ G. Jiang, DJ Hill, M. Kowalczuk, B. Johnston, G. Adamus, V. Irorere, I. Radecka: Carbon Sources for Polyhydroxyalkanoates and an Integrated Biorefinery. In: International Journal of Molecular Sciences . Volume 17, number 7, July 2016, p., Doi : 10.3390 / ijms17071157 , PMID 27447619 , PMC 4964529 (free full text) (review).
- ↑ Elodie Bugnicourt, Patrizia Cinelli, Vera Alvarez, Undrea Lazzeri: Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging . In: eXPRESS Polymer Letters . 8, No. 11, 2014, pp. 791-808. doi : 10.3144 / expresspolymlett.2014.82 .
- ^ Bio-on chairman charged with market manipulation and false accounting - bioplastics MAGAZINE. Retrieved November 18, 2019 .