Propinal
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Propinal | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 3 H 2 O | ||||||||||||||||||
| Brief description |
colorless to brown liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 54.05 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
= 1.4065 g cm −3 at 20 ° C |
||||||||||||||||||
| boiling point | |||||||||||||||||||
| solubility |
soluble in water, ethanol , diethyl ether , benzene , toluene , acetone , in chloroform and methanol |
||||||||||||||||||
| Refractive index |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Propinal is an organic compound with the empirical formula C 3 H 2 O. It is the simplest chemical compound of an aldehyde with a C≡C triple bond and belongs to the class of alkynals .
Occurrence and representation
The occurrence of propinal in addition to the C 3 H 2 O isomers cyclopropenone and propadienone H 2 C = C = C = O was detected in molecular clouds in interstellar space .
The chemical synthesis of propargylaldehyde was first reported by Ludwig Claisen in 1898 . Starting from 2,3-dibromopropionaldehyde diethylacetal (from acrolein by addition of bromine and acetalization with triethyl orthoformate ), double dehydrobromination (elimination of hydrogen bromide HBr) forms propinal diethylacetal, which is split with sulfuric acid to give free propinal.
A laboratory specification from Organic Syntheses describes the production of propinal by oxidation of propargyl alcohol with toxic, mutagenic and carcinogenic chromium (VI) oxide and sulfuric acid in a modest yield of 35 to 41%. In an improved variant with methyl ethyl ketone MEK as the solvent for propynol and adding an aqueous solution of chromium trioxide / sulfuric acid at room temperature, a yield of 91% is achieved. Because of the problematic properties of chromium trioxide and the difficult processing and disposal of Cr (VI) salts, this synthesis route is no longer up-to-date.
Vacuum pyrolysis of the easily accessible dipropargyl ether (accessible in a Williamson ether synthesis from propynol and propargyl bromide ) at 750 ° C. to propinal (80% yield) and propadiene also appears suitable for larger batches .
properties
As a pure substance, propinal is a colorless liquid that dissolves in water and many organic solvents. When standing for a long time, the connection changes color from yellow to brown-red. According to L. Claisen, “propargylaldehyde is a liquid that irritates the nose and eyes just as violently or even more than acrolein”. Traces of peroxides or bases can initiate an explosive polymerization of propargylaldehyde stored in glass containers. Therefore propinal should be used with caution in a maximum of 10% solution in higher boiling solvents, e.g. B. toluene, handled and stored in aluminum or plastic storage containers.
Applications
Additions to the triple bond
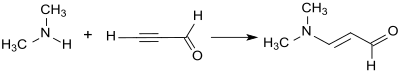
The addition of dimethylamine to the triple bond of propargylaldehyde in the sense of a vinylation according to Reppe produces 3-dimethylaminoacrolein in 88% yield as a yellow oil.
In a similar way, nucleophiles with hydroxyl groups or thiol groups add to propinal to form 3-substituted acroleins which have a double bond conjugated with a carbonyl group in the molecule.
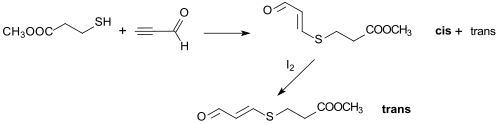
For example, the Michael addition of methyl 3-mercaptopropionate to propinal produces a cis / trans isomer mixture of a 3-thio-substituted acrolein, since treatment with iodine can completely convert it into the more stable trans isomer.
In a [1,4] addition, propinal, which is both stabilized and activated by inclusion in zeolite Y cage structures, reacts with less reactive methoxybenzenes, such as. B. anisole to the flavoring 4-methoxycinnamaldehyde or in a [2 + 2] cycloaddition with unreactive cycloalkenes , such as. B. Cyclohexene to the bicyclic unsaturated aldehyde bicyclo [4.2.0] oct-7-en.
Additions to the carbonyl group
Grignard compounds easily add to the carbonyl group of propargylaldehyde, with the corresponding secondary alcohols being formed.
Cycloadditions with propinal
The 1,3-dipolar cycloaddition of azides , e.g. B. Trimethylsilyl azide (CH 3 ) 3 SiN 3 on propargylaldehyde gives 4-formyl-1,2,3-triazole in useful yield.
1,2-Diaminobenzenes condense on heating in a methanol / dimethylformamide mixture with cyclization to give tetraaza [14] annulenes , the heavy metal ions, such as. B. manganese 3+ , nickel 2+ or copper 2+ , complex and can serve as model compounds for porphyrins .
Individual evidence
- ↑ a b c d e Propynal. Retrieved October 28, 2019 .
- ↑ a b M.G. Veliev, MM Guseinov: An improved synthesis of propynal . In: Synthesis . tape 6 , 1980, pp. 461 , doi : 10.1055 / s-1980-29052 .
- ↑ a b c L. Claisen: propargylaldehyde and phenylpropargylaldehyde . In: Ber. German Chem. Ges. Volume 31 , no. 1 , 1898, p. 1021-1023 , doi : 10.1002 / cber.189803101185 .
- ↑ a b c Patrick Perlmutter: Propargyl Aldehyde . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2001, doi : 10.1002 / 047084289X.rp262m .
- ^ A b Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Oxford, UK 2015, ISBN 978-0-323-28659-6 , pp. 24 .
- ↑ a b J.C. Sour: Propiolaldehyde In: Organic Syntheses . 36, 1956, p. 66, doi : 10.15227 / orgsyn.036.0066 ; Coll. Vol. 4, 1963, p. 813 ( PDF ).
- ↑ J.-C. Louisen et al .: The interstellar chemistry of H2C3O isomers . In: Monthly Notices of the Astronomical Society . tape 456 , no. 4 , 2016, p. 4101–4110 , doi : 10.1093 / mnras / stv2866 ( arxiv.org [PDF]).
- ^ VK Ahluwalia, R. Aggarwal: Organic Syntheses: Special Techniques . Alpha Science International Ltd., Pangbourne, UK 2001, ISBN 1-84265-058-0 , pp. 27-28 .
- ^ RE Geiger, M. Lalonde, H. Stoller, K. Schleich: Cobalt-catalyzed cycloadditions of alkynes and nitriles to pyridines: A new approach to pyridoxine (vitamin B6) . In: Helv. Chim. Acta . tape 67 , no. 5 , 1984, pp. 1274-1282 , doi : 10.1002 / hlca.19840670513 .
- ↑ H. McNab, G. Morel, E. Stevenson: A short, convenient synthesis of propynal . In: J. Chem. Res. (S) . tape 6 , 1997, pp. 207 , doi : 10.1039 / A700453B .
- ↑ F. Wille, L. Saffer, W. Weißkopf: On the knowledge of propargylaldehyde I: preparation, polymerization and reaction with amines . In: Justus Liebigs Ann. Chem. Band 568 , no. 1 , 1950, p. 34-46 , doi : 10.1002 / jlac.19505680103 .
- ↑ D. Makula, P. Lamy: Sécurité: Stabilité de l'aldehyde propargylique (propynal) . In: L'actualité chimique . Juin-Juillet, No. 103 , 1983, pp. 31-34 (www.lactualitechimique.org/numero/103).
- ↑ Patent DE944852 : Process for the preparation of derivatives of 3-amino-acrolein. Registered on August 25, 1955 , published on June 28, 1956 , applicant: Badische Anilin- & Soda-Fabrik AG, inventor: F. Wille.
- ↑ R. Hanko, MD Hammond, R. Fruchtmann, J. Pfitzner, GA Place: Design, synthesis, and 5-lipoxygenase-inhibiting properties of 1-thiosubstituted butadienes . In: J. Med. Chem. Volume 33 , no. 4 , 1990, pp. 1163-1170 , doi : 10.1021 / jm00166a013 .
- ↑ D. Hayashi, Y. Igura, Y. Masui, M. Onaka: Stabilization and activation of unstable propynal in the zeolite nanospace and its application to addition reactions . In: Catal. Sci. Technol. tape 19 , no. 7 , 2017, p. 4422-4440 , doi : 10.1039 / C7CY01161J .
- ↑ MM Demina, PS Novopashin, GI Sarapulova, LI Larina, AS Smolin, VS Fundamenskii, AA Kashev, AS Medvedeva: 1,3-Dipolar cycloadditions of trimethylsilyl azide to propynals and dimerization of 1 H -1,2,3-triazole- 5-carbaldehydes to tricyclic bis-hemiaminals . In: Russian J. Org. Chem. Band 40 , no. 12 , 2004, p. 1804-1809 , doi : 10.1007 / s11178-005-0103-4 .
- ↑ K. Sakata, H. Nakamura, M. Hashimoto: Preparation and characterization of isothiocyanatomanganese (III) complexes of tetraaza [14] annulenes . In: Inorg. Chim. Acta . tape 83 , no. 3 , 1984, pp. L67-L70 , doi : 10.1016 / S0020-1693 (00) 82375-0 .