Thymine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Thymine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 5 H 6 N 2 O 2 | ||||||||||||||||||
| Brief description |
white, crystalline solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 126.04 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.46 g cm −3 |
||||||||||||||||||
| Melting point |
316-317 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Thymine (T, Thy, 5-Methyluracil) is one of the four nucleobases in DNA , along with adenine , cytosine and guanine . In the RNA there is uracil in its place . It is a heterocyclic organic compound with a pyrimidine backbone and three substituents ( oxygen atoms in positions 2 and 4, methyl group in position 5). The nucleosides of thymine are the deoxythymidine in DNA and the rare ribothymidine in RNA (e.g. in tRNA ). In the Watson-Crick base pairing , it forms two hydrogen bonds with adenine.
Presentation and extraction
Isolation can be made from beef brains or cod roe.
A synthetic preparation is possible by cyclizing N-ethoxycarbonyl-3-methoxy-2-methylacrylamide in aqueous ammonia solution.
Another synthesis starts from 3-methyl malic acid , which is decarboxylated in fuming sulfuric acid and condensed with urea .
properties
Physical Properties
Thymine forms shiny, bitter-tasting, needle-shaped or prism-shaped crystals that melt at 335–337 ° C with decomposition. The compound dissolves well in hot water, but the solubility in alcohol and ether is poor. In alkaline media it dissolves with salt formation as a result of enolate formation derived from the enol form 5-methyl-2,4- pyrimidinediol .
Chemical properties
In principle, thymine can exist in six tautomeric structures. The lactam form (1) is preferred over the enol forms.
History and biological meaning
In 1893, the later Nobel Prize winner Albrecht Kossel reported a discovery. He and his assistant Albert Neumann had extracted nucleic acid from the calf's thymus glands and treated them with sulfuric acid. A well-crystallized cleavage product was formed, for which the name thymine - derived from the thymus gland - was suggested.
Thymine can be part of DNA or various nucleosides and nucleotides .
Nucleosides
Via the N 1 atom of the ring, thymine can be bound to the C 1 atom of deoxyribose N -glycosidically ; one then speaks of a nucleoside , the deoxythymidine . The nucleoside ribothymidine is formed when it binds to ribose .

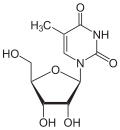
Deoxythymidine , dT Ribothymidine , T.
Nucleotides
The phosphorylation of thymidine at the C 5 atom of ribose leads to the important nucleotides deoxythymidine monophosphate (dTMP), deoxythymidine diphosphate (dTDP) and deoxythymidine triphosphate (dTTP).
Part of the DNA
In the DNA double helix , thymine forms two hydrogen bonds with the corresponding adenine base of the complementary strand via the 4-oxo group and the N 3 -H group .
Comparison of thymine and uracil
Thymine takes the place of uracil in DNA . Uracil can be formed relatively easily from cytosine by deamination and hydrolysis , which then changes (mutates) the base sequence and possibly changes the information genetically encoded in the nucleotide sequence .
Thymine, on the other hand, differs from uracil in that it has an additional methyl group and so cannot easily arise from cytosine. Uracil present in the DNA can thus be recognized as a mutation and exchanged for cytosine by base excision repair .
Thymine dimer
Thymine dimers are DNA mutations that are induced by UV radiation . Two thymine bases lying next to one another on a DNA strand combine covalently via a [2 + 2] cycloaddition to form a dimer, which is a relatively stable cyclobutane derivative.
Skin cells exposed to sunlight are particularly susceptible to such a mutation. For this reason, thymine dimers are discussed as a major cause of the development of skin cancer .
use
Thymine is used as a starting material for some drugs such as B. zidovudine , telbivudine, and clevudine .
Related links

|
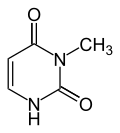
|
| Uracil | 3-methyluracil |
Individual evidence
- ↑ a b c d data sheet thymine at AlfaAesar, accessed on November 23, 2013 ( PDF )(JavaScript required) .
- ↑ K. Ozeki, N. Sakabe, J. Tanaka: "The crystal structure of thymine", in: Acta Cryst. , 1969 , B25 , p. 1038 ( doi : 10.1107 / S0567740869003505 ).
- ↑ Shimizu: Biochem. Journal , 1921 , 117 , p. 262.
- ↑ König, Grossfeld: Biochem. Journal , 1913 , 54 , p. 371.
- ^ G. Shaw, RN Warrener: "33. Purines, pyrimidines, and glyoxalines. Part VIII. New syntheses of uracils and thymines “, in: J. Chem. Soc. , 1958 , pp. 157-161 ( doi : 10.1039 / jr9580000157 ).
- ↑ HW Scherp: "Convenient Syntheses of Thymine and 5-Methylisocytosine", in: J. Am. Chem. Soc. , 1946 , 68 , pp. 912-913 ( doi : 10.1021 / ja01209a510 ).
- ^ A b Brockhaus ABC Chemie , Verlag Harri Deutsch Frankfurt / Main and Zurich 1965.
- ↑ a b entry on thymine. In: Römpp Online . Georg Thieme Verlag, accessed on December 9, 2014.
- ^ S. Hauptmann, J. Gräfe, H. Remane: Textbook of Organic Chemistry , VEB Deutscher Verlag der Grundstoffindustrie, Leipzig 1980, p. 556.
- ^ Kossel, A. and A. Neumann: About thymine, a cleavage product of nucleic acid. In: Reports of the German Chemical Society , Volume 26, 1893, p. 2753
- ^ Kossel, A. and A. Neumann: Presentation and cleavage products of nucleic acid (adenylic acid). Lecture in: Reports of the German Chemical Society , Volume 27, 1894, p. 2215.
- ^ K. Peter C. Vollhardt , Neil E. Schore: Organische Chemie , 4th edition, Wiley-VCH, Weinheim 2005, ISBN 3-527-31380-X , p. 181.
- ^ Alberts , Bray, Johnson, Lewis: Textbook of Molecular Cell Biology , 2nd corrected edition, Wiley-VCH, Weinheim 2001, ISBN 3-527-30493-2 .
Web links
- Entry for Thymine in the Human Metabolome Database (HMDB) , accessed November 18, 2013.






