Vinylene carbonate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Vinylene carbonate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 2 O 3 | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 86.05 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.355 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
19-22 ° C |
|||||||||||||||
| boiling point |
162 ° C |
|||||||||||||||
| Refractive index |
1.421 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−459.9 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Vinylene carbonate or 1,3-dioxol-2-one , VC for short, is the simplest unsaturated cyclic carbonic acid ester . Vinylene carbonate can also be understood as the cyclic carbonate of the hypothetical ( Z ) -ethene-1,2-diol. The activated double bond in this five-membered oxygen-containing heterocycle makes the molecule a reactive monomer for homopolymerization and copolymerization and a dienophile in Diels-Alder reactions . In highly pure form, vinylene carbonate is a colorless, stable solid below room temperature.
presentation
Ethylene carbonate has been the common starting material for vinylene carbonate since it was first described in 1953. In a UV-initiated photochlorination reaction with chlorine or sulfuryl chloride at 60-70 ° C in substance, monochlorethylene carbonate is formed in the first stage, which in the second stage is produced by dehydrochlorination , e.g. B. with triethylamine , optionally diluted with ethylene carbonate, vinylene carbonate or MTBE , is converted into vinylene carbonate at 40-60 ° C.
Instead of in the liquid phase, the dehydrochlorination can also be carried out in the gas phase on a contact impregnated with zinc chloride in a fluidized bed reactor at 350-500 ° C. with average yields of 69% of theory. be performed. The reaction route, which appears simple, only gives yields of 70 to 80% of theory because of a large number of side reactions. of impure end product. In the case of the chlorination of ethylene carbonate in bulk or in solution, for example, a. 2-chloroacetaldehyde, polychlorinated ethylene carbonates and, with ring opening, other chlorine-containing compounds whose separation from the end product by distillation using a thin-film evaporator , using fractional recrystallization or zone melting is very expensive. The content of by-products can also be reduced by stirring with sodium borohydride or urea at an elevated temperature. Another problem is the pronounced thermal stability of VC, which decomposes within minutes at temperatures above 80 ° C. By optimizing the chlorination conditions to suppress the formation of by-products and a combination of several gentle cleaning processes, high-purity vinylene carbonate can be produced in yields of over 70% of theory. can be obtained. The tendency of the liquid VC to polymerize is reduced by adding inhibitors, such as. B. butylated hydroxytoluene (BHT), suppressed.
properties
In industrial production, vinylene carbonate is usually obtained as a yellow to brown liquid. A solid which melts at 20–22 ° C and has a chlorine content of less than 10 ppm can be obtained through suitable process management and cleaning steps. Liquid VC quickly turns yellow even when light is excluded and must be stabilized by adding radical scavengers. In solid form, the highly pure substance is long-term stable when stored below 10 ° C. Vinylene carbonate dissolves in a variety of solvents, such as ethanol , tetrahydrofuran , ethylene carbonate , propylene carbonate and other dipolar aprotic electrolyte solvents for rechargeable lithium-ion batteries , such as dimethyl carbonate , diethyl carbonate and the like. Ä.
use
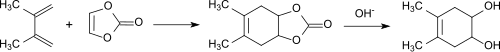
In the first publication on vinylene carbonate, Diels-Alder reactions were described using the example of addition of 2,3-dimethylbutadiene to form the bicyclic carbonate and subsequent hydrolysis to form cis-4,5-dihydroxy-1,2-cyclohexene.
With cyclopentadiene as the diene, the vicinal norbornene diol bicyclo [2.2.1] hept-5-ene-2,3-diol is formed after hydrolysis . The Swern oxidation to the 1,2-ketone bicyclo [2.2.1] hept-5-en-2,3-dione proceeds in the variant with trifluoroacetic anhydride instead of oxalyl chloride with a yield of 73% of theory.
When exposed to UV radiation, ketones react with vinylene carbonate to form bicyclic exo- oxetanes .
Vinylene carbonate reacts with phosphorus (V) sulfide to form the corresponding vinyl thionocarbonate (2-thiono-1,3-dioxol-4-en) which, when exposed to UV radiation, gives ketene in quantitative yield . The reaction is a good alternative to the decomposition of α-diazoketones.
Vinylene carbonate is more widely used as an electrolyte additive for lithium ion batteries, which promotes the formation of a film that is insoluble in the electrolyte as a solid interface between the negative electrode and the electrolyte (SEI, solid electrolyte interphase). This polymer film enables ion conduction, but prevents the reduction of the electrolyte on the negative (graphite) electrode and contributes significantly to the long-term stability of lithium ion batteries.
Recent publications suggest that the cyclic sultone 3-fluoro-1,3-propane sultone (FPS) has superior properties to vinylene carbonate as an SEI generator.
Since the 1,3-propane sultone on which the FPS is based is classified as a particularly dangerous carcinogenic substance, it must be assumed that FPS is also a significant hazard.
Polymers
The first work on vinylene carbonate already describes bulk polymerization to give colorless polymers that were water-soluble after hydrolysis. Later publications suggest that the first authors only produced oligomers with low molar mass . The purity of the monomeric vinylene carbonate is decisive for the production of polymers with higher molar mass and useful properties. Vinylene carbonate can be used in bulk, in solution, in suspension and in dispersion with the help of radical starters , such as. B. azobis (isobutyronitrile) (AIBN) or benzoyl peroxide , homopolymerized or copolymerized with other vinyl monomers such as vinyl pyrrolidone or vinyl propionate .
Polyvinylene carbonate is readily soluble in acetone and dimethylformamide . The solutions obtained, however, tend to decompose even at room temperature. In the patent literature, tensile fibers, clear, colorless and mechanically resilient films, membranes for reverse osmosis , and supports for affinity chromatography made of polyvinylene carbonate are described.
In addition to the instability in solutions, the tendency of the polyvinylene carbonate to hydrolyze is problematic even in a weakly alkaline medium. The splitting of the cyclic carbonate ring creates polyhydroxymethylene (PHM) with the repeating unit - (CHOH) -, whose behavior is much more similar to cellulose than the related polyvinyl alcohol with the repeating unit - (CH 2 -CHOH) -.
For example, films made of polyhydroxymethylene, which are obtained by alkaline hydrolysis of polyvinylene carbonate films using sodium methoxide in methanol , are crystalline and show high tensile strengths . Similar to cellulose, polyhydroxymethylene can be dissolved in hot sodium hydroxide solution and converted into a highly swellable polymer through crosslinking, which can absorb up to 10,000 times its weight in water. Polyhydroxymethylene is soluble in anhydrous hydrazine and can be converted into cellulose-like fibers by spinning in water. Similar to cellulose, polyhydroxymethylene reacts with carbon disulfide in the alkaline to form a xanthate , from which water-insoluble polyhydroxymethylene is obtained again by precipitation in dilute sulfuric acid.
The lack of more recent literature, in particular patent literature, on poly-VC and PHM suggests that the properties of the homo- and coolymers obtained and the molded parts produced from them could not meet the expectations made therein.
safety instructions
Due to its problematic toxicological and ecotoxicological profile and its potentially carcinogenic properties, vinylene carbonate requires special care when handling.
See also
Individual evidence
- ↑ a b c d e f g h i j Datasheet Vinylene carbonate from Sigma-Aldrich , accessed on December 22, 2013 ( PDF ).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-23.
- ↑ a b c M.S. Newman, RW Addor: Vinylene Carbonate . In: Journal of the American Chemical Society . tape 75 , no. 5 , 1953, pp. 1263-1264 , doi : 10.1021 / ja01101a526 .
- ↑ a b c Patent US6395908 : Process for the preparation of vinylene carbonate, and the use thereof. Published on 28 May 2002 , Applicant: Merck Patent Gesellschaft, inventor B. Seifert et al ..
- ↑ Patent EP1881972 : Process for producing vinylene carbonate. Published on August 28, 2013 , applicant: Saltigo GmbH, inventors: Reinhard Langer, Anke Beckmann, Paul Wagner, Heinrich Grzinia, Marielouise Schneider, Ulrich Notheis, Lars Rodefeld, Nikolaus Müller.
- ↑ a b Patent US8022231 : Process for preparing monochloroethylene carbonate and subsequent conversion to vinylene carbonate. Published on September 20, 2011 , Applicant: Evonik Degussa GmbH, inventor M. Lerm et al ..
- ↑ Patent EP1881973 : PROCESS FOR PRODUCING CARBONATE vinylene. Published on January 30, 2008 , inventor: R. Langer et al ..
- ↑ Patent GB899205 : The purification and polymerization of vinylene carbonate. Published June 20, 1962 , Applicant: ICI Ltd., Inventor: BF Nesbitt, I. Goodman.
- ↑ Morris Zief, Hollister Ruch, Charles H. Schramm: Low temperature zone refining apparatus . In: Journal of Chemical Education . tape 40 , no. 7 , 1963, pp. 351 , doi : 10.1021 / ed040p351 .
- ↑ a b c N. D. Field, JR Schaefgen: High molecular weight poly (vinylene carbonate) and derivatives . In: Journal of Polymer Science A: Polymer Chemistry . tape 58 , no. 166 , 1962, pp. 533-543 , doi : 10.1002 / pol . 1962.1205816630 .
- ↑ PCT application WO 2006/119910, method for purifying vinylene carbonate , inventor: R. Langer et al., Applicant: Lanxess Deutschland GmbH, published on November 16, 2006.
- ↑ Patent EP1881971 : High-purity vinylene carbonate and a method of purifying vinylene carbonate. Published on January 30th, 2008 , applicant: Saltigo GmbH, inventor: Reinhard Langer, Paul Wagner, Heinrich Grzinia.
- ↑ Patent WO2006119908 : METHOD OF STORING AND TRANSPORTING VINYLENE CARBONATE. Published on November 16, 2006 , applicant: Lanxess Deutschland GmbH, inventor: R. Langer.
- ↑ T. Kobayashi, S. Kobayashi: Swern Oxidation of Bicyclo [2.2.1] hept-5-ene-2,3-diol and Its Pyrazine-fused Derivatives: An Improved Synthesis of Bicyclo [2.2.1] hept-5- ene-2,3-dione and An Unexpected Ring-Opening Reaction . In: Molecules . tape 5 , no. 9 , 2000, pp. 1062-1067 , doi : 10.3390 / 50901062 .
- ↑ Hans-Michael Fischler, Willy Hartmann: Note on the preparation of vinyl thione carbonate and some alkyl and aryl-substituted derivatives . In: Chemical Reports . tape 105 , no. 8 , 1972, p. 2769-2771 , doi : 10.1002 / cber.19721050838 .
- ↑ Handbook of Reagents for Organic Syntheses, Sulfur-Containing Reagents , ed. LA Paquette, Wiley-VCH, 2010, ISBN 978-0-470-74872-5 , p. 535.
- ↑ Hsiang-Hwan Lee, Yung-Yun Wang, Chi-Chao Wan, Mo-Hua Yang, Hung-Chun Wu, Deng-Tswen Shieh: The function of vinylene carbonate as a thermal additive to electrolyte in lithium batteries . In: Journal of Applied Electrochemistry . tape 35 , no. 6 , 2005, p. 615–623 , doi : 10.1007 / s10800-005-2700-x .
- ↑ M. Broussely et al., Main aging mechanisms in Li ion batteries , J. Power Sources, 146 (1), 90-96 (2005), doi: 10.1016 / j.jpowsour.2005.03.172 .
- ↑ Patent DE102004018929 : Electrolyte composition and its use as an electrolyte material for electrochemical energy storage systems. Published on November 17, 2005 , Applicant: Applicant Degussa AG, inventor: V. Hennige et al ..
- ↑ HM Jung et al., Fluoropropane sultone as an SEI-forming additive that outperforms vinylene carbonate , J. Mater. Chem. A, 1, 11975-11981 (2013), doi: 10.1039 / C3TA12580G .
- ↑ a b Patent US2993030 : Process for polymerizing vinylene carbonate. Published July 16, 1961 , Applicant: JT Baker Chemical Co., Inventor: GE Ham, M. Zief.
- ↑ M. Krebs, C. Schneider, Vinylene carbonate - A study of its polymerization and copolymerization behavior , Adv. Chem., 142 (9), 92-98 (1975), doi: 10.1021 / ba-1975-0142.ch009 .
- ↑ Patent GB899205 : The purification and polymerization of vinylene carbonate. Published June 20, 1962 , Applicant: Imperial Chemical Industries Ltd, Inventor: Brenda Frances Nesbitt, Isaac Goodman.
- ↑ Patent US4098771 : Process for the preparation of polymers of vinylene carbonate. Published on July 4, 1978 , applicant: Hoechst AG, inventor: H. Huemer, K. Burg.
- ↑ J. Huang et al., Investigations on vinylene carbonate I. Preparation and properties of poly- (vinylene carbonate) , Chinese J. Polym. Sci., 8 (3), 197-203 (1990).
- ↑ Patent US3332894 : Polyvinyl carbonate desalination membrane and a method of producing the same. Published July 25, 1967 , Inventors: PA Cantor, RE Kesting.
- ↑ Patent US4788278 : Polyvinylene carbonate and polyhydroxymethylene, processes for their preparation and their use. Published on November 29, 1988 , Applicant: Hoechst AG, Inventor: O. Mauz.
- ↑ Patent US4061692 : Process for the manufacture of swellable, absorptive polymers of polyhydroxy methylene. Published on December 6, 1977 , inventors: A. Holst, M. Kostrzewa.
- ↑ Patent US4076680 : Poly (hydroxymethylene) solutions. Published on February 28, 1978 , inventor: MK Akkapeddi, HK Reimschuessel.
- ↑ Patent US3331800 : Preparation of solutions of polyhydroxymethylene-containing polymers. Published on July 18, 1967 , inventor H. Schübelweiher et al ..
- ↑ Entry on Vinylene Carbonate at TCI Europe, accessed on January 5, 2014.