Tartrazine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tartrazine | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 16 H 9 N 4 Na 3 O 9 S 2 | ||||||||||||||||||
| Brief description |
orange powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 534.37 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tartrazine ( E 102 ) is a synthetic azo dye that is used as a lemon-yellow to orange-colored food coloring . The fabric is colourfast .
history
Tartrazine was developed in 1884 by the Swiss chemist Johann Heinrich Ziegler (1857–1936) in the laboratories of the Bindschedler factory for the chemical industry in Basel ( CIBA ) and patented by BASF in Germany in 1885 (DRP 34294). In 1887 it was published in the reports of the German Chemical Society . The original synthesis was carried out by reacting tetrahydroxysuccinic acid with phenylhydrazine-4-sulfonic acid . For the connection, Ziegler formulated the disodium salt of an osazone :
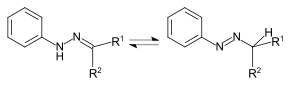
In 1893, Ziegler patented an alternative manufacturing process (British patent 5693). Starting from the consideration that a hydrazone a tautomeric form of an azo compound is ( azo-hydrazo tautomerism ), he succeeded in the synthesis of tartrazine by azo coupling of diazotized sulfanilic acid with the reaction product of Oxalessigsäureethylester with phenylhydrazine-4-sulfonic acid and subsequent alkaline hydrolysis .
In 1897 Richard Anschütz was able to show that the structure published by Ziegler is not correct, but that the compound is a pyrazolone derivative .
Structure of tartrazine in the hydrazo and tautomeric azo form
Initially, tartrazine was used as a lightfast dye for wool .
Manufacturing
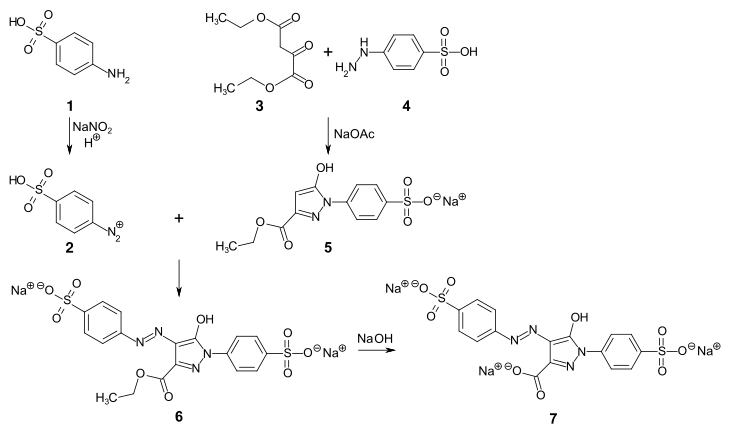
In the first synthesis of tartrazine 3 , phenylhydrazine-4-sulfonic acid 1 was reacted with tetrahydroxysuccinic acid 2 under alkaline conditions :
In the alternative synthesis that is still common today, sulfanilic acid 1 is diazotized and the diazonium salt 2 is coupled to the pyrazolone compound 5 . This is obtained by condensation of phenylhydrazine-4-sulfonic acid with ethyl oxaloacetate in the presence of sodium acetate . The ester 6 is then saponified to tartrazine 7 with sodium hydroxide solution .
use
Tartrazine is used for liqueurs, spirits, wines, non-alcoholic beverages, fizzy and fizzy powder , bubble teas , baked goods, confectionery, snacks, pudding powder , dessert dishes, mustard, processed cheese , fish and crab pastes, in wasabi imitation, as a colored coating for coated tablets Coloring of cheese rinds and artificial casings and used for medicines.
health
The substance is allergenic and therefore problematic for allergy sufferers. It can cause breathing difficulties, rashes, hay fever , blurred vision, and skin spots. Since no antibodies can be detected, one speaks of a pseudo-allergy . Consumer advice centers advise against consumption and the children's cancer clinic at the University of Düsseldorf considers the substance to be dangerous. A cross allergy to benzoic acid or acetylsalicylic acid (aspirin) is known.
Tartrazine is discussed as a cause of hyperactivity . This suspicion has been confirmed by recent studies.
The authors Knieriemen-Suter / Knieriemen write in their book Cosmetic Ingredients from A to Z: The critical advisor that this dye is suspected of "releasing aromatic amines that are classified as carcinogenic or toxic."
Legal situation
In the European Union , tartrazine is a conditionally approved additive for certain foods. With the exception of beverages with more than 1.2% alcohol, foods to which E 102 has been added must also be labeled with the statement “May impair activity and attention in children”. In Germany and Austria , previous bans were lifted by EU law; In Austria the ban even applied to everyday objects.
In Germany, tartrazine is according to the additive approval regulation (ZZulV) for certain foods such as fine baked goods, confectionery or ice cream, candied fruits (up to 200 mg / kg), curry powder (up to 500 mg / kg), mustard (up to 300 mg / kg) , Smoked fish and canned peas (each up to 100 mg / kg) and many drinks are permitted, but not for meat or sausage - except for edible sausage casings. Therefore, the inadmissible, i.e. forbidden, lightening of meat or sausage with E 102, especially to simulate higher freshness, as well as the placing on the market of such products (without adequate labeling) is a criminal offense.
Tartrazine is banned in Norway . In the US, the fabric is known as FD&C Yellow No. 5 approved for certain foods, pharmaceuticals and cosmetics. In Spain and France, tartrazine can be found in supermarkets as a food coloring.
Tartrazine can be replaced by a mixture of quinoline yellow (E 104) and yellow orange S (E 110).
Individual evidence
- ↑ a b Entry for E 102: Tartrazine in the European database on food additives, accessed on June 27, 2020.
- ↑ Tartrazine data sheet from AlfaAesar, accessed on March 30, 2010 ( PDF )(JavaScript required) .
- ↑ a b c Entry on tartrazine. In: Römpp Online . Georg Thieme Verlag, accessed on May 21, 2014.
- ↑ a b Tartrazine data sheet from Sigma-Aldrich , accessed on April 23, 2011 ( PDF ).
- ↑ a b c Entry on tartrazine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ SD LOCKEY: Allergic reactions due to FD and C Yellow No. 5, tartrazine, an aniline dye used as a coloring and identifying agent in various steroids. In: Annals of allergy. Volume 17, Sep-Oct 1959, pp. 719-721, PMID 14417794 .
- ↑ a b FAO Nutrition Meetings Report Series. Vol. 38B, p. 88, 1966.
- ^ A b c Johann Heinrich Ziegler: About the Tartrazine, a new class of dyes . In: Reports of the German Chemical Society . tape 20 , no. 1 , 1887, p. 834 ff ., doi : 10.1002 / cber.188702001188 .
- ↑ External identifiers or database links for tetrahydroxysuccinic acid : CAS number: 76-30-2, EC number: 200-951-3, ECHA InfoCard: 100.000.866 , PubChem : 6439 , ChemSpider : 6199 , Wikidata : Q27291294 .
- ↑ External identifiers or database links for phenylhydrazine-4-sulfonic acid : CAS number: 98-71-5, EC number: 202-694-2, ECHA InfoCard: 100.002.450 , PubChem : 66825 , ChemSpider : 60189 , Wikidata : Q27277588 .
- ↑ External identifiers from or database links to Oxalessigsäureethylester : CAS number: 108-56-5, EC number: 203-594-1, ECHA -InfoCard: 100003268 , PubChem : 66951 , ChemSpider : 60310 , Wikidata : Q27251741 .
- ^ Johann Heinrich Ziegler: 104. Necrologist . In: Quarterly publication of the Natural Research Society in Zurich . tape 81 , no. 3-4 , 1936, pp. 313–314 ( ngzh.ch [PDF]): "It is not the place here to describe this second synthesis, only let us say that ZIEGLER was one of the first to recognize the so-called tautomerism of organic compounds and immediately put it to practical use."
- ↑ a b c R. Anschütz: About the constitution of tartrazine . In: Justus Liebig's Annals of Chemistry . tape 294 , no. 2 , 1897, p. 219 , doi : 10.1002 / jlac.18972940207 .
- ↑ Donna McCann, Angelina Barrett et al. a .: Food additives and hyperactive behavior in 3-year-old and 8/9-year-old children in the community: a randomized, double-blinded, placebo-controlled trial. In: The Lancet. 370, 2007, p. 1560, doi : 10.1016 / S0140-6736 (07) 61306-3 .
- ↑ EFSA : Assessment of the results of the study by McCann et al. (2007) on the effect of some colors and sodium benzoate on children's behavior. (PDF; 437 kB) The EFSA Journal (2008) 660, 1–53.
- ↑ Helene Knieriemen-Suter, Heinz Knieriemen: Cosmetic ingredients from A to Z: The critical advisor . AT Verlag, Aarau 2005, ISBN 978-3-85502974-7 , p. 94.
- ↑ Art. 4 Regulation (EC) No. 1333/2008 of the European Parliament and of the Council of December 16, 2008 on food additives, with Annex II.
- ↑ Appendix 5 to Art. 24 Regulation (EC) No. 1333/2008 of December 16, 2008 on food additives (PDF) , accessed on November 16, 2019.
- ↑ Appendix 1 Part B and Part C to § 3 of the Ordinance on the Approval of Additives to Food for Technological Purposes (Additive Approval Ordinance - ZZulV).
- ↑ § 6 Paragraph 1 and Paragraph 2 and § 59 Paragraph 1 No. 1 - no. 3 Food and Feed Code (LFGB) through the use of a non-approved coloring agent, furthermore in the event of a violation of the prohibition of deception in accordance with Section 11 (2) no. 2 LFGB according to § 59 para. 1 no. 9 LFGB.