Ribociclib: Difference between revisions
Anypodetos (talk | contribs) →top: ChEMBL |
Anypodetos (talk | contribs) Pharmacokinetics; remove outdated information |
||
| Line 8: | Line 8: | ||
| tradename = Kisqali |
| tradename = Kisqali |
||
| Drugs.com = {{Drugs.com|parent|kisqali}} |
| Drugs.com = {{Drugs.com|parent|kisqali}} |
||
| MedlinePlus = |
| MedlinePlus = a617008 |
||
| pregnancy_AU = <!-- A/B1/B2/B3/C/D/X --> |
| pregnancy_AU = <!-- A/B1/B2/B3/C/D/X --> |
||
| pregnancy_AU_comment = |
| pregnancy_AU_comment = |
||
| pregnancy_US = <!-- A/B/C/D/X/N --> |
| pregnancy_US = <!-- A/B/C/D/X/N --> |
||
| pregnancy_category= |
| pregnancy_category= |
||
| routes_of_administration = Oral |
| routes_of_administration = [[Oral administration|By mouth]] (tablets) |
||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> |
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> |
||
| legal_AU_comment = |
| legal_AU_comment = |
||
| Line 24: | Line 24: | ||
| legal_status = |
| legal_status = |
||
<!-- Pharmacokinetic data --> |
<!-- Pharmacokinetic data --> |
||
| bioavailability = |
| bioavailability = Unknown |
||
| protein_bound = ~70% |
| protein_bound = ~70% |
||
| metabolism = Liver ([[CYP3A4]]) |
| metabolism = Liver ([[CYP3A4]]) |
||
| Line 31: | Line 31: | ||
| elimination_half-life = 32.0 (29.7–54.7) hrs |
| elimination_half-life = 32.0 (29.7–54.7) hrs |
||
| duration_of_action= |
| duration_of_action= |
||
| excretion = 69% feces, |
| excretion = 69% feces, 23% urine |
||
<!-- Identifiers --> |
<!-- Identifiers --> |
||
| CAS_number = 1211441-98-3 |
| CAS_number = 1211441-98-3 |
||
| Line 57: | Line 57: | ||
==Medical uses== |
==Medical uses== |
||
Ribociclib was approved by the [[US FDA]] in March 2017 and the [[European Medicines Agency]] in August 2017 for use in combination with an [[aromatase inhibitor]] to treat [[Hormone receptor|HR-positive]], [[HER2/neu|HER2-negative]] advanced or metastatic breast cancers.<ref name=kf>[http://www.genengnews.com/gen-news-highlights/fda-clears-novartis-kisqali-for-combination-breast-cancer-therapy/81254019 FDA Clears Novartis Kisqali for Combination Breast Cancer Therapy. March 2017]</ref><ref name="EPAR">{{cite web|url=http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004213/WC500233997.pdf|title=Kisqali: EPAR – Product Information|publisher=[[European Medicines Agency]]|date=2017-08-31}}</ref> |
Ribociclib was approved by the [[US FDA]] in March 2017 and the [[European Medicines Agency]] in August 2017 for use in combination with an [[aromatase inhibitor]] (such as [[letrozole]]) to treat [[Hormone receptor positive breast tumor|HR-positive]], [[HER2/neu|HER2-negative]] advanced or metastatic breast cancers.<ref name=kf>[http://www.genengnews.com/gen-news-highlights/fda-clears-novartis-kisqali-for-combination-breast-cancer-therapy/81254019 FDA Clears Novartis Kisqali for Combination Breast Cancer Therapy. March 2017]</ref><ref name="EPAR">{{cite web|url=http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004213/WC500233997.pdf|title=Kisqali: EPAR – Product Information|publisher=[[European Medicines Agency]]|date=2017-08-31}}</ref> |
||
==Side effects== |
==Side effects== |
||
| Line 74: | Line 74: | ||
When used in combination with other drugs such as an [[Anaplastic lymphoma kinase|ALK]] or an [[Mitogen-activated protein kinase kinase|MEK]] inhibitor, ribociclib has been shown to have a synergistic effect, resulting in improved responses.<ref>{{cite journal |first1=Jeffrey Alan |last1=Sosman |first2=Muaiad |last2=Kittaneh |first3=Martijn P. J. K. |last3=Lolkema |first4=Michael Andrew |last4=Postow |first5=Gary |last5=Schwartz |first6=Catherine |last6=Franklin |first7=Alessandro |last7=Matano |first8=Suraj |last8=Bhansali |first9=Sudha |last9=Parasuraman |first10=Kevin |last10=Kim |year=2014 |title=A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with ''NRAS''-mutant melanoma: Early encouraging clinical activity |journal=Journal of Clinical Oncology |volume=32 |issue=15 Suppl |pages=9009 |url=http://hwmaint.meeting.ascopubs.org/cgi/content/abstract/32/15_suppl/9009 }}</ref><ref>{{cite journal |last1=Wood |first1=Andrew C. |last2=Krytska |first2=Kateryna |last3=Ryles |first3=Hannah |last4=Sano |first4=Renata |last5=Li |first5=Nanxin |last6=King |first6=Frederick |last7=Smith |first7=Timothy |last8=Tuntland |first8=Tove |last9=Kim |first9=Sunkyu |last10=Caponigro |first10=Giordano |last11=He |first11=You Qun |last12=Jennifer |first12=Harris |last13=Mosse |first13=Yael |title=Abstract 1000: Combination CDK4/6 and ALK inhibition demonstrates on-target synergy against neuroblastoma |journal=Cancer Research |volume=74 |issue=19 Supplement |year=2014 |pages=1000 |doi=10.1158/1538-7445.AM2014-1000 }}</ref> Again, this is likely a result of "[[crosstalk (biology)|crosstalk]]" between signaling pathways. Simply blocking one pathway in cancer [[tumorigenesis]] can sometimes result in "tumor compensation", where the tumor compensates for the blocked signaling pathway by utilizing other pathways to survive. By blocking several pathways at once, it is thought that the tumor is less able to compensate, and a greater anti-tumor response is often observed. It is also of interest that utilizing ribociclib in combination with other agents has been shown to reduce the development of resistance to these agents.<ref name=Samson2014/> In other words, cancer’s development of [[drug resistance]] can be mitigated with the addition of ribociclib to the therapeutic regime. |
When used in combination with other drugs such as an [[Anaplastic lymphoma kinase|ALK]] or an [[Mitogen-activated protein kinase kinase|MEK]] inhibitor, ribociclib has been shown to have a synergistic effect, resulting in improved responses.<ref>{{cite journal |first1=Jeffrey Alan |last1=Sosman |first2=Muaiad |last2=Kittaneh |first3=Martijn P. J. K. |last3=Lolkema |first4=Michael Andrew |last4=Postow |first5=Gary |last5=Schwartz |first6=Catherine |last6=Franklin |first7=Alessandro |last7=Matano |first8=Suraj |last8=Bhansali |first9=Sudha |last9=Parasuraman |first10=Kevin |last10=Kim |year=2014 |title=A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with ''NRAS''-mutant melanoma: Early encouraging clinical activity |journal=Journal of Clinical Oncology |volume=32 |issue=15 Suppl |pages=9009 |url=http://hwmaint.meeting.ascopubs.org/cgi/content/abstract/32/15_suppl/9009 }}</ref><ref>{{cite journal |last1=Wood |first1=Andrew C. |last2=Krytska |first2=Kateryna |last3=Ryles |first3=Hannah |last4=Sano |first4=Renata |last5=Li |first5=Nanxin |last6=King |first6=Frederick |last7=Smith |first7=Timothy |last8=Tuntland |first8=Tove |last9=Kim |first9=Sunkyu |last10=Caponigro |first10=Giordano |last11=He |first11=You Qun |last12=Jennifer |first12=Harris |last13=Mosse |first13=Yael |title=Abstract 1000: Combination CDK4/6 and ALK inhibition demonstrates on-target synergy against neuroblastoma |journal=Cancer Research |volume=74 |issue=19 Supplement |year=2014 |pages=1000 |doi=10.1158/1538-7445.AM2014-1000 }}</ref> Again, this is likely a result of "[[crosstalk (biology)|crosstalk]]" between signaling pathways. Simply blocking one pathway in cancer [[tumorigenesis]] can sometimes result in "tumor compensation", where the tumor compensates for the blocked signaling pathway by utilizing other pathways to survive. By blocking several pathways at once, it is thought that the tumor is less able to compensate, and a greater anti-tumor response is often observed. It is also of interest that utilizing ribociclib in combination with other agents has been shown to reduce the development of resistance to these agents.<ref name=Samson2014/> In other words, cancer’s development of [[drug resistance]] can be mitigated with the addition of ribociclib to the therapeutic regime. |
||
===Pharmacokinetics=== |
|||
==Clinical trials== |
|||
The percentage of ribociclib absorbed in the gut has not been determined. Highest [[blood plasma]] levels are reached after one to four hours; and after repeated dosage, steady state concentrations are reached after about eight days. Food intake has no effect on absorption rates. When in the bloodstream, about 70% of ribociclib are bound to [[plasma protein]]s.<ref name="Drugs.com" /><ref name="EPAR" /> |
|||
Ribociclib has been shown to be well-tolerated, although its best response as a single agent as of 2014 has been stable disease.<ref>{{cite journal |first1=B. |last1=Geoerger |first2=F. |last2=Bourdeaut |first3=S.G. |last3=Dubois |first4=M.D. |last4=Dewire |first5=A. |last5=Marabelle |first6=A.D. |last6=Pearson |first7=S. |last7=Modak |first8=R. |last8=Kan |first9=A. |last9=Matano |first10=S.G. |last10=Bhansali |first11=S. |last11=Parasuraman |first12=S.N. |last12=Chi |year=2014 |title=455P Phase I study of LEE011 (CDK4/6 inhibitor) in patients with malignant rhabdoid tumors, neuroblastoma, and cyclin D–CDK4/6 pathway-activated tumors |journal=Annals of Oncology |volume=25 |issue=suppl 4 |pages=iv151–2 |doi=10.1093/annonc/mdu331.15 |url=http://annonc.oxfordjournals.org/content/25/suppl_4/iv151.3.short }}</ref> In October 2016, good results (increased [[progression-free survival]]) were reported from the MONALEESA-2 trial (in combination with [[letrozole]]) in metastatic [[breast cancer]].<ref>[http://www.medpagetoday.com/hematologyoncology/BreastCancer/62304 Anti-CDK4/6 Boosts PFS in Metastatic Breast Cancer. Oct 2016]</ref> |
|||
The substance is mainly metabolized by CYP3A4 and subsequently by various [[Phase II metabolism|phase II enzymes]], resulting in a large number of metabolites. Those with highest blood plasma concentrations in humans are called CCI284 (an unspecified ''[[Nitrogen|N]]''-[[hydroxylation]] product), LEQ803 (the ''N''-[[demethylation]] product) and M1 (a [[glucuronide]]). All metabolites have negligible clinical activity.<ref name="Drugs.com" /><ref name="EPAR" /> |
|||
Ribociclib is eliminated with an average [[biological half-life]] of 32 hours, mostly (69%) via the feces, but also (23%) via the urine. The unchanged drug accounts for 17% of the substance in the feces and 12% of the substance in the urine, the rest being metabolites.<ref name="Drugs.com" /><ref name="EPAR" /> |
|||
==Research== |
|||
{{as of|2017|09}}, ribociclib is in phase II development for several indications, including [[liposarcoma]],<ref>{{ClinicalTrialsGov|NCT03096912|A Study Assessing Efficacy & Safety of Ribociclib in Patients With Advanced Well/Dedifferentiated Liposarcoma}}</ref> [[endometrial carcinoma]]<ref>{{ClinicalTrialsGov|NCT03008408|Study of Ribociclib (LEE011), Everolimus, and Letrozole, in Patients With Advanced or Recurrent Endometrial Carcinoma}}</ref> and [[neuroendocrine tumors]] of the [[foregut]].<ref>{{ClinicalTrialsGov|NCT02420691|LEE011 in Neuroendocrine Tumors of Foregut Origin}}</ref> |
{{as of|2017|09}}, ribociclib is in phase II development for several indications, including [[liposarcoma]],<ref>{{ClinicalTrialsGov|NCT03096912|A Study Assessing Efficacy & Safety of Ribociclib in Patients With Advanced Well/Dedifferentiated Liposarcoma}}</ref> [[endometrial carcinoma]]<ref>{{ClinicalTrialsGov|NCT03008408|Study of Ribociclib (LEE011), Everolimus, and Letrozole, in Patients With Advanced or Recurrent Endometrial Carcinoma}}</ref> and [[neuroendocrine tumors]] of the [[foregut]].<ref>{{ClinicalTrialsGov|NCT02420691|LEE011 in Neuroendocrine Tumors of Foregut Origin}}</ref> |
||
| Line 83: | Line 88: | ||
==See also== |
==See also== |
||
*[[Palbociclib]], similar mechanism and indications |
*[[Palbociclib]], a drug with similar mechanism and indications |
||
==References== |
==References== |
||
Revision as of 09:02, 9 September 2017
 | |
| Clinical data | |
|---|---|
| Trade names | Kisqali |
| Other names | LEE 011 |
| AHFS/Drugs.com | kisqali |
| MedlinePlus | a617008 |
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Protein binding | ~70% |
| Metabolism | Liver (CYP3A4) |
| Elimination half-life | 32.0 (29.7–54.7) hrs |
| Excretion | 69% feces, 23% urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.234.566 |
| Chemical and physical data | |
| Formula | C23H30N8O |
| Molar mass | 434.548 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
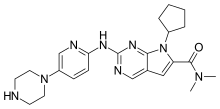
Ribociclib (trade name Kisqali[1]) is an inhibitor of cyclin D1/CDK4 and CDK6, and is used for the treatment of certain kinds of breast cancer.[2] It is also being studied as a treatment for other drug-resistant cancers.[3] It was developed by Novartis and Astex Pharmaceuticals.[4]
Medical uses
Ribociclib was approved by the US FDA in March 2017 and the European Medicines Agency in August 2017 for use in combination with an aromatase inhibitor (such as letrozole) to treat HR-positive, HER2-negative advanced or metastatic breast cancers.[1][5]
Side effects
The most common side effects in studies were decreased blood cell counts, mainly neutropenia (in 75% of patients, as compared to 5% under placebo), but also anemia (18% vs. 5%). Gastrointestinal disorders were also common, for example nausea (52% vs. 29%) and diarrea (35% vs. 22%), as was alopecia (33% vs. 16%). The drug also increases QT time and liver enzymes (alanine transaminase, aspartate transaminase).[2][5]
Interactions
As ribociclib is mainly metabolized by the liver enzyme CYP3A4, inhibitors of this enzyme increase its concentrations in the body and could potentiate side effects and toxicity. Examples of such inhibitors include ketoconazole and similar antifungal drugs, ritonavir, clarithromycin, as well as grapefruit. Conversely, drugs that induce CYP3A4, such as rifampicin and St John's Wort, can decrease ribociclib concentrations.[2][5]
Ribociclib itself is a moderate to strong CYP3A4 inhibitor and therefore can increase concentrations of other drugs that share this metabolism, as has been shown with midazolam. It also inhibits a number of transporter proteins and could thus theoretically interfere with the transport of other drugs. It could also amplify QT prolongation of other drugs such as antiarrhythmics, clarithromycin, and haloperidol.[2][5]
Pharmacology
Mechanism of action
Cyclin-dependent kinases (CDKs) 4 and 6 are enzymes that have been shown to promote cell division and multiplication in both normal and cancer cells. Many cancer cells have shown abnormalities that increase the activity of CDK, leading to the inactivation of certain tumor suppressor genes.[3][6] This has led to the idea that inhibiting CDK4 will slow the growth of tumors by reactivating these tumor suppressors.
When used in combination with other drugs such as an ALK or an MEK inhibitor, ribociclib has been shown to have a synergistic effect, resulting in improved responses.[7][8] Again, this is likely a result of "crosstalk" between signaling pathways. Simply blocking one pathway in cancer tumorigenesis can sometimes result in "tumor compensation", where the tumor compensates for the blocked signaling pathway by utilizing other pathways to survive. By blocking several pathways at once, it is thought that the tumor is less able to compensate, and a greater anti-tumor response is often observed. It is also of interest that utilizing ribociclib in combination with other agents has been shown to reduce the development of resistance to these agents.[3] In other words, cancer’s development of drug resistance can be mitigated with the addition of ribociclib to the therapeutic regime.
Pharmacokinetics
The percentage of ribociclib absorbed in the gut has not been determined. Highest blood plasma levels are reached after one to four hours; and after repeated dosage, steady state concentrations are reached after about eight days. Food intake has no effect on absorption rates. When in the bloodstream, about 70% of ribociclib are bound to plasma proteins.[2][5]
The substance is mainly metabolized by CYP3A4 and subsequently by various phase II enzymes, resulting in a large number of metabolites. Those with highest blood plasma concentrations in humans are called CCI284 (an unspecified N-hydroxylation product), LEQ803 (the N-demethylation product) and M1 (a glucuronide). All metabolites have negligible clinical activity.[2][5]
Ribociclib is eliminated with an average biological half-life of 32 hours, mostly (69%) via the feces, but also (23%) via the urine. The unchanged drug accounts for 17% of the substance in the feces and 12% of the substance in the urine, the rest being metabolites.[2][5]
Research
As of September 2017[update], ribociclib is in phase II development for several indications, including liposarcoma,[9] endometrial carcinoma[10] and neuroendocrine tumors of the foregut.[11]
Chemistry
Ribociclib is used in form of its tartrate salt. It is a slightly hygroscopic yellow to brown crystalline powder that is soluble in aqueous acids.[12]
See also
- Palbociclib, a drug with similar mechanism and indications
References
- ^ a b FDA Clears Novartis Kisqali for Combination Breast Cancer Therapy. March 2017
- ^ a b c d e f g FDA Professional Drug Information on Kisqali. Accessed 2017-09-08.
- ^ a b c Samson, Kurt (2014). "LEE011 CDK Inhibitor Showing Early Promise in Drug-Resistant Cancers". Oncology Times. 36 (3): 39–40. doi:10.1097/01.COT.0000444043.33304.c1.
- ^ "Novartis LEE011 (ribociclib) granted FDA Priority Review for first-line treatment of HR+/HER2- advanced breast cancer". Novartis. 2016-11-01.
- ^ a b c d e f g "Kisqali: EPAR – Product Information" (PDF). European Medicines Agency. 2017-08-31.
- ^ Kim, S.; Loo, A.; Chopra, R.; Caponigro, G.; Huang, A.; Vora, S.; Parasuraman, S.; Howard, S.; Keen, N.; Sellers, W.; Brain, C. (2014). "Abstract PR02: LEE011: An orally bioavailable, selective small molecule inhibitor of CDK4/6- Reactivating Rb in cancer". Molecular Cancer Therapeutics. 12 (11_Supplement): PR02. doi:10.1158/1535-7163.TARG-13-PR02.
- ^ Sosman, Jeffrey Alan; Kittaneh, Muaiad; Lolkema, Martijn P. J. K.; Postow, Michael Andrew; Schwartz, Gary; Franklin, Catherine; Matano, Alessandro; Bhansali, Suraj; Parasuraman, Sudha; Kim, Kevin (2014). "A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: Early encouraging clinical activity". Journal of Clinical Oncology. 32 (15 Suppl): 9009.
- ^ Wood, Andrew C.; Krytska, Kateryna; Ryles, Hannah; Sano, Renata; Li, Nanxin; King, Frederick; Smith, Timothy; Tuntland, Tove; Kim, Sunkyu; Caponigro, Giordano; He, You Qun; Jennifer, Harris; Mosse, Yael (2014). "Abstract 1000: Combination CDK4/6 and ALK inhibition demonstrates on-target synergy against neuroblastoma". Cancer Research. 74 (19 Supplement): 1000. doi:10.1158/1538-7445.AM2014-1000.
- ^ Clinical trial number NCT03096912 for "A Study Assessing Efficacy & Safety of Ribociclib in Patients With Advanced Well/Dedifferentiated Liposarcoma" at ClinicalTrials.gov
- ^ Clinical trial number NCT03008408 for "Study of Ribociclib (LEE011), Everolimus, and Letrozole, in Patients With Advanced or Recurrent Endometrial Carcinoma" at ClinicalTrials.gov
- ^ Clinical trial number NCT02420691 for "LEE011 in Neuroendocrine Tumors of Foregut Origin" at ClinicalTrials.gov
- ^ "Kisqali: EPAR – Public assessment report" (PDF). European Medicines Agency. 2017-08-31.
