Camptothecin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
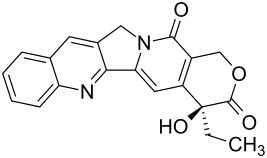
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Camptothecin | ||||||||||||||||||
| other names |
( S -) 4-ethyl-4-hydroxy-1 H -pyrano [3 ', 4': 6,7] indolizino [1,2- b ] quinoline-3,14- (4 H , 12 H ) -dione ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 20 H 16 N 2 O 4 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 348.36 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
260 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Camptothecin is a cell-toxic , naturally occurring monoterpenoid, pentacyclic alkaloid . It consists of a quinoline and an isoquinoline ring system . The substance was discovered in 1966 as part of a screening for naturally occurring potential cancer therapeutics by Monroe E. Wall and Mansukh C. Wani .
The chemically to the quinoline alkaloids scoring Camptothecin inhibits the enzyme topoisomerase I and showed in clinical trials , a cytostatic effect. However, due to unfavorable physicochemical and pharmacological properties, camptothecin is not used in cancer therapy. Further developments of camptothecin, topotecan and irinotecan , are approved for the treatment of malignant tumors.
The total synthesis succeeded Ekkehard Winterfeldt in 1972 .
Occurrence
Camptothecin is isolated from the bark and roots, the seeds and the wood, as well as (young) leaves of Camptotheca acuminata, the Chinese tree of happiness, a Chinese tree belonging to the tupelo family (Nyssaceae) . The alkaloid can also be detected in the other parts of this plant. The highest content of camptothecin is found in the young leaves and flower buds. The alkaloid content is somewhat lower in the fruits and seeds, roots and in the bark.
Apart from its occurrence in Camptotheca acuminata , camptothecin could sporadically be detected in individual representatives of hardly related families within the seed plants . There was positive evidence in Pyrenacantha klaineana , Merrilliodendron megacarpum , Nothapodytes foetida ( Icacinaceae ), Ophiorrhiza spp. ( Rubiaceae ) as well as Tabernaemontana alternifolia (Syn .: Ervatamia heyneana ) and Mostuea brunonis ( Gelsemiaceae ). Camptothecin can also be produced in root cultures , so-called hairy root cultures , by Camptotheca acuminata or Ophiorrhiza pumila .
biosynthesis
Although camptothecin does not have an indole structure , but a quinoline structure, camptothecin belongs biogenetically to the iridoid indole alkaloids . Like all representatives of this group, camptothecin is also derived from the amino acid tryptophan and secologanin , which comes from the terpene metabolism . After the intermediate formation of strictosidine and enzymatic oxidation of the ring E derived from secolaganin, a rearrangement to camptothecin takes place as the end product of the biosynthesis.
pharmacology
Mechanism of action
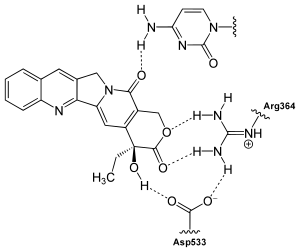
Camptothecin is a topoisomerase I inhibitor, the physiological function of which is to reduce the shear stress of the DNA double strand. The alkaloid mediates this inhibitory effect via a simultaneous binding to topoisomerase I and to the DNA bound to the enzyme with the aid of hydrogen bonds . In the interaction with topoisomerase I, on the one hand, particularly the ring E of camptothecin, which interacts with the amino acid residues aspartic acid -533 and arginine -364 of the enzyme, is involved. On the other hand, the lactam - oxygen of the ring D for interaction with the DNA, primarily by cytosine occurs groups in position +1 responsible. Camptothecin thus stabilizes the topoisomerase I-DNA complex and thus prevents re- closure (re- ligation ) of the DNA after it has been unwound. Since the DNA is not closed again, not only single-strand breaks, but also double-strand breaks are possible. These lead to programmed cell death (apoptosis). The effectiveness of camptothecin is limited to the S phase of the cell cycle , in which DNA synthesis also takes place.
chemistry
Stereochemistry
Camptothecin is a chiral drug with a stereocenter in position 20 in ring E. An S configuration is essential for its pharmacological effect ; R- camptothecin is pharmacologically inactive.
Structure-activity relationships
In order to optimize the cytostatic effectiveness of camptothecin and at the same time reduce its undesirable pharmacological and physicochemical properties, numerous modifications were made to the molecular structure. The S -configured hydroxyl group in position 20, the 2-pyridone element in ring D, the lactone structure in ring E and the extensive planarity of the entire ring system have proven to be essential for cytostatic effectiveness . Changes to these parameters lead to a drastic loss of effectiveness.
Ring system A / B
The most promising changes in the molecular structure of camptothecin concern the substituents on rings A and B. These also play a decisive role in the therapeutically used camptothecin derivatives irinotecan and topotecan. Monosubstitution or disubstitution at positions 9 and 10 with electron-rich substituents such as OH, NH 2 or halogen groups , like monosubstitution at position 11, leads to an increase in effectiveness. Disubstitution in positions 10 and 11, on the other hand, is associated with a loss of effectiveness. An exception to this rule are the hexacyclic camptothecin derivatives such as lurtotecan , which are linked to one another at positions 10 and 11 via methylenedioxy or ethylenedioxy groups and have an increased topoisomerase I-inhibiting effect. A substitution at position 12 is generally associated with a drastic loss of effectiveness. The solubility and the affinity for DNA can be modulated via the substituent in position 7.
Ring system C / D
Significantly more critical than the described substitutions on the quinoline ring system are the substitutions on rings C and D. Substitutions in positions 5 and 14 generally lead to a reduction in the topoisomerase I-inhibitory activity.
Ring system E
Ring E allows considerably more modifications. Replacing the hydroxyl group with an amino or halogen group in position 20, which interacts with the lactone group via an intramolecular hydrogen bond and which is responsible for the relative instability of the lactone ring, leads to a loss of effectiveness. The hydroxyl group in position 20 is also seen as an essential proton donor for an interaction with topoisomerase I via intermolecular hydrogen bonds. Esterification of this group, as in the case of the polymer conjugates protecan and pegamotecan , leads to prodrugs with increased stability of the lactone ring, reduced in vitro cytotoxicity and possibly increased in vivo tumor activity. Alternatively, ring expansion with the formation of a β-hydroxylactone results not only in an increase in the physicochemical stability but also in an increase in the topoisomerase I-inhibiting effect.
Derivatives
Based on camptothecin, structural modifications have led to the development of new drugs (e.g. atiratecan ) that have been tested in clinical studies and, as in the case of topotecan and irinotecan, have been approved for the chemotherapy of malignant tumors. The structural modifications relate in particular to the substituents of the quinoline ring system. Topotecan is approved as a second-line therapeutic for ovarian cancer and small cell lung cancer . Irinotecan is approved as a first-line therapeutic in combination with the standard therapeutic agents 5-fluorouracil and folinic acid for the treatment of colon cancer . Lurtotecan and exatecan have been tested in Phase II and III clinical trials.
Esterification of the hydroxyl group in position 20 and linkage with polyethylene glycol (PEG) lead to polymer conjugates that are used for the targeted release of active ingredients at the site of action ( drug targeting ). Examples of this are the drugs pegamotecan and protecan, which are currently in clinical trials.
Individual evidence
- ↑ a b c d data sheet (S) - (+) - Camptothecin from Sigma-Aldrich , accessed on March 15, 2011 ( PDF ).
- ^ ME Wall, MC Wani, CE Cook, KH Palmer, AI McPhail, GA Sim: Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from camptotheca acuminate . In: J. Am. Chem. Soc . 88, 1966, pp. 3888-3890. doi : 10.1021 / ja00968a057 .
- ^ A. Lorence, CL Nessler: Camptothecin, over four decades of surprising findings . In: Phytochemistry . 65, No. 20, 2004, pp. 2735-2749. doi : 10.1016 / j.phytochem.2004.09.001 . PMID 15474560 .
- ↑ Y. Yamazaki, M. Kitajima, M. Arita et al .: Biosynthesis of camptothecin. In silico and in vivo tracer study from [1-13C] glucose . In: Plant Physiol . 134, No. 1, 2004, pp. 161-170. doi : 10.1104 / pp.103.029389 . PMID 14657405 . PMC 316296 (free full text).
- ↑ DJ Adams, ML Wahl, JL Flowers, B. Sen, M. Colvin, MW Dewhirst, G. Manikumar, MC Wani: Camptothecin analogs with enhanced activity against human breast cancer cells. II. Impact of the tumor pH gradient . In: Cancer Chemotherapy and Pharmacology . 57, No. 2, 2005, pp. 145-154. doi : 10.1007 / s00280-005-0008-5 .
- ↑ MR Redinbo, L. Stewart, P. Kuhn, JJ Champoux, WGJ Hol: Crystal structure of human topoisomerase I in covalent and noncovalent complexes with DNA . In: Science . 279, 1998, pp. 1504-1513. doi : 10.1126 / science.279.5356.1504 .
- ^ RP Verma, C. Hansch: Camptothecins: a SAR / QSAR study . In: Chem Rev. . 109, No. 1, 2009, pp. 213-235. doi : 10.1021 / cr0780210 .
- ↑ QY Li, YG Zu, RZ Shi, LP Yao: Review camptothecin: current perspectives . In: Curr. Med. Chem . 13, No. 17, 2006, pp. 2021-2039. doi : 10.2174 / 092986706777585004 . PMID 16842195 .
- ↑ L. Lesueur-Ginot, D. Demarquay, R. Kiss et al .: Homocamptothecin, an E-ring modified camptothecin with enhanced lactone stability, retains topoisomerase I-targeted activity and antitumor properties . In: Cancer Res . 59, No. 12, 1999, pp. 2939-2943. PMID 10383158 .